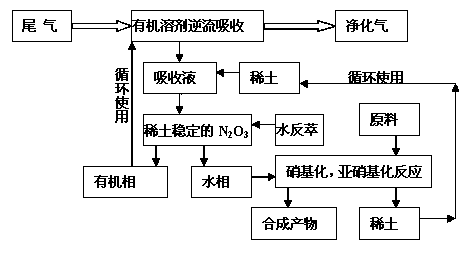
New method for treating nitrogen oxide waste gas
A nitrogen oxide and nitric oxide technology, applied in chemical instruments and methods, nitrous oxide capture, separation methods, etc., can solve problems that do not conform to the law of chemical reactions, achieve the effect of improving absorption efficiency and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Determination of absorption solvent and its absorption capacity
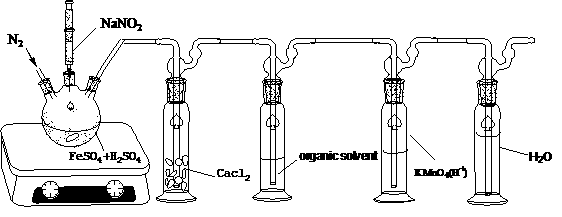
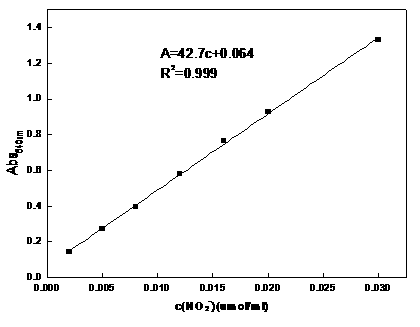
[0043] use figure 2 In the first absorption bottle, organic solvents such as esters, alcohols or ketones are put into the first absorption bottle to absorb NO, and the NO content in the exhaust gas passing through the absorption bottle is detected to determine the effect of the solvent in the first absorption bottle on NO. The absorption effect, the method is to oxidize the tail gas through an acidic potassium permanganate solution to oxidize the incompletely absorbed NO into NO 2 , NO 2 After being dissolved in water, it can be absorbed by the water in the second absorption bottle, and its content can be detected by Griess reagent method. The method is under acidic conditions, nitrite NO 2 - It can undergo diazotization reaction with Griess reagent (p-aminobenzenesulfonic acid, N-(1-naphthalene) ethylenediamine, hydrochloric acid) to generate pink diazo compound, which has the maximum ...
Embodiment 2
[0048] Embodiment 2; Absorption of NO and NO with water and alkaline aqueous solution 2 Comparison of the effect of mixed gas
[0049] 2 mmol NaNO 2 Reaction under acidic conditions to produce 1:1 NO and NO 2 , through the primary absorption bottle 100ml water, 100ml NaOH solution (containing NaOH 20mmol) or 100ml Y-MS-butyl acetate (Y-MS concentration is 0.1mol / L, the same below), the secondary absorption bottle is 100ml Y-MS- Butyl acetate, then the gas that has not been absorbed by the primary absorption bottle will be absorbed in the secondary absorption bottle, and the subsequent reaction will cause it to develop a blue-green color. From the degree of color development, it can be judged that the primary absorption liquid absorbs NO-NO 2 s efficiency. When the absorption solution is water and NaOH solution, after the gas absorption is complete, take 0.5ml for Griess color development. The results are shown in Table 2, it can be seen that butyl acetate has a significant...
Embodiment 3
[0053] The MS-Y-BA solution is used as the NO absorption solution, and the reaction device is not removed after the absorption is complete, so that the whole system is placed in an oxygen-free environment. After directly heating the absorption bottle for a period of time, no blue-green color is formed. It shows that the oxidation of NO can only be carried out in the presence of oxygen. After taking part of the liquid from the stopcock at the bottom of the absorption bottle and heating it in air for a few minutes, the color of the solution changed, initially light yellow, then green, and finally blue-green. It shows that the color reaction is an aerobic reaction, that is, the oxygen content is the controlling factor mentioned in Example 1. In order to further prove that the color development process requires oxygen to oxidize NO into NO 2 , we directly put NO and NO 2 (by NaNO 2 obtained by reaction under acidic conditions) into the Y-MS-butyl acetate mixed solution to see ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com