Piperidine derivative, and preparation method and application thereof in preparation of halofuginone
A kind of derivative, piperidine technology, applied in the synthesis field of alkaloid halofuginone hydrobromide, can solve the problems of affecting the final yield, increasing the difficulty of industrialization, increasing the cost of equipment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Preparation of Cbz-protected 5-aminopental (Ⅱ)
[0056]
[0057] Weigh 14.52g (0.1mol) of 5-aminovaleryl (I), 16.8mL (0.12mol) of triethylamine, put them together in a 500mL three-necked flask, add 60mL of dichloromethane solvent; 17.92 g (0.105 mol) of benzyl chloroformate dissolved in 20 mL of dichloromethane was added dropwise thereto, and the reaction was stirred at room temperature (25° C.) for 10 hours. Stop the reaction, transfer the reaction solution into a separatory funnel, add 80mL of water, mix well, let stand to separate layers, wash the organic layer with saturated ammonium chloride aqueous solution (80mL×2), dry the organic phase with anhydrous magnesium sulfate, and evaporate under reduced pressure. The organic phase was dried to obtain 27.64 g of Cbz-protected 5-aminovaleryl (II), with a yield of 99%.
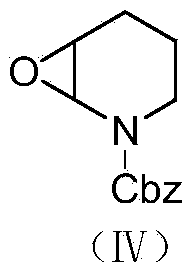
[0058] (2) Preparation of benzyl N-formate-2,3-epoxypiperidine (Ⅳ)
[0059]
[0060] Weigh 27.93g (0.1mol) of Cbz-protected 5-aminovaleryl (II...
Embodiment 2
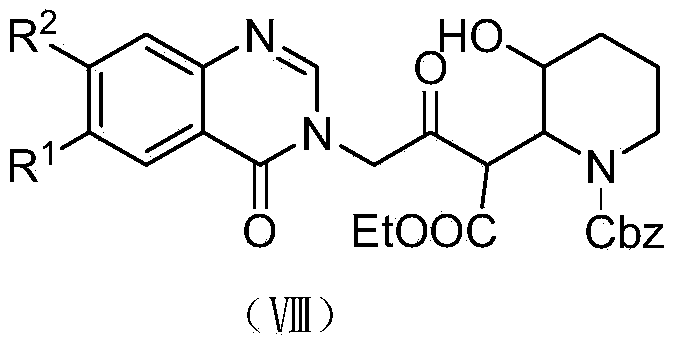
[0076] (1) Preparation of 4-(4-oxo-3H-quinazolin-3 base)-3-oxo-butanoic acid ethyl ester (VII-2)
[0077]
[0078] Add 7.3g (0.05mol) of 3H-quinazolin-4-one (Ⅴ-2), 8.28g (0.06mol) of potassium carbonate, and 60mL of DMF into a 250ml round bottom flask. Stir the reaction at room temperature for half an hour, add 10.45 g (0.05 mol) of ethyl bromoacetoacetate, and react at 70° C. for 8 hours under a nitrogen atmosphere. Stop the reaction, evaporate the solvent, then extract with 70mL ethyl acetate, and wash with saturated sodium chloride solution (60mL×3). The organic layer was collected, dried over anhydrous magnesium sulfate, filtered and evaporated to remove the solvent by a rotary evaporator. The intermediate 4-(4-oxo-3H-quinazolin-3 base)-3-oxo-butanoic acid was separated by silica gel column chromatography (mobile phase dichloromethane:methanol=100:1-2, volume ratio) Ethyl ester (VII-2) 13.18g, yield 96.2%. The structural characterization data are as follows:
[0079...
Embodiment 3
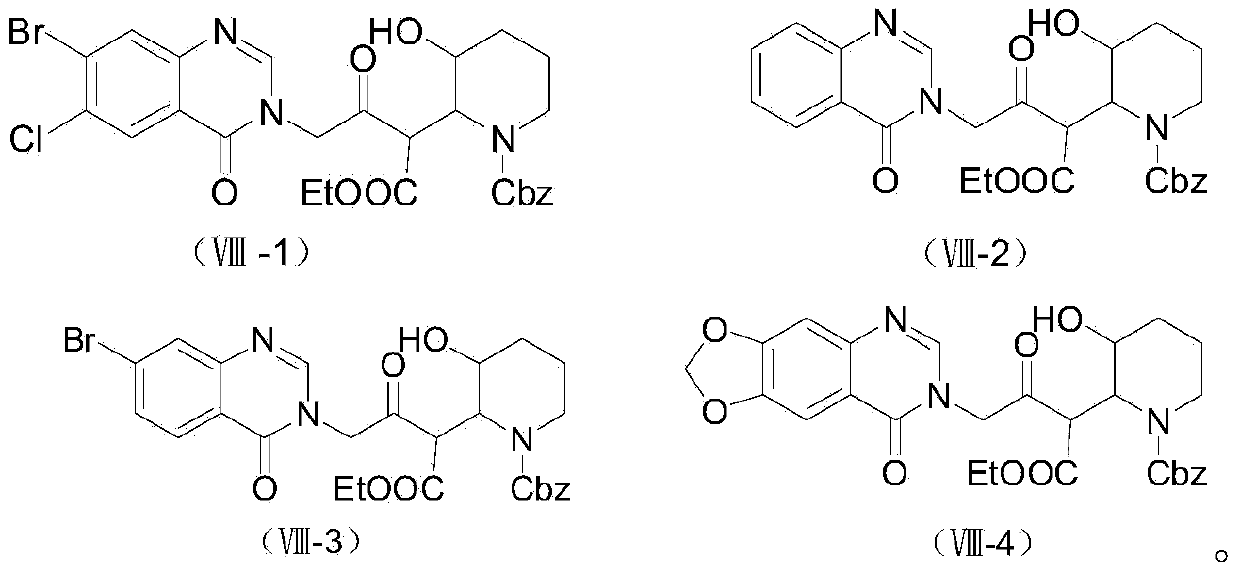
[0089] (1) Preparation of 4-(7-bromo-4-carbonyl-3H-quinazolin-3 base)-3-oxo-butanoic acid ethyl ester (VII-3)
[0090]
[0091] Add 11.25g (0.05mol) of 7-bromo-3H-quinazolin-4-one (Ⅴ-3), 8.28g (0.06mol) of potassium carbonate, and 65mL of DMF into a 250ml round bottom flask. Stir the reaction at room temperature for half an hour, add 10.45 g (0.05 mol) of ethyl bromoacetoacetate, and react at 70° C. for 8 hours under a nitrogen atmosphere. Stop the reaction, evaporate the solvent, then extract with 80×2mL ethyl acetate, and wash with saturated sodium chloride solution (60mL×3). The organic layer was collected, dried over anhydrous magnesium sulfate, filtered and evaporated to remove the solvent by a rotary evaporator. The intermediate 4-(7-bromo-4-carbonyl-3H-quinazolin-3 base)-3- Oxo-butyric acid ethyl ester (VII-3) 16.9 g, yield 95.8%. The structural characterization data are as follows:
[0092] 1 H NMR (500MHz, TMS, CD 3Cl)δ8.36(s,1H),7.90(s,1H),7.73-7.71(m,1H),7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com