Anode for electrowinning and electrowinning method using same
An electrolytic extraction and anode technology, which is applied in the direction of electrodes, electrolytic processes, electrolytic components, etc., can solve the problems of hindering the reaction, increasing the electrolytic voltage, and increasing the electrolytic voltage, so as to improve the catalytic activity, reduce the electrolytic voltage, and reduce the electrolytic voltage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
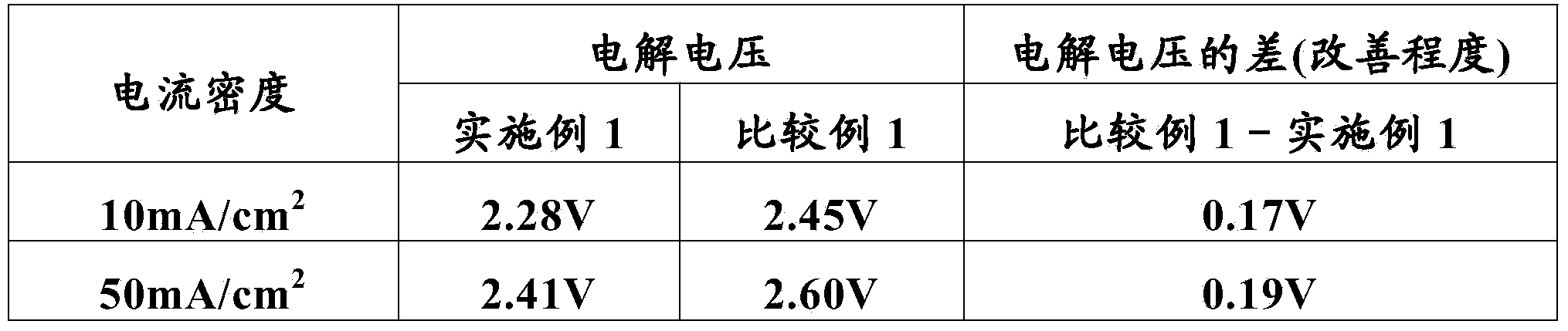
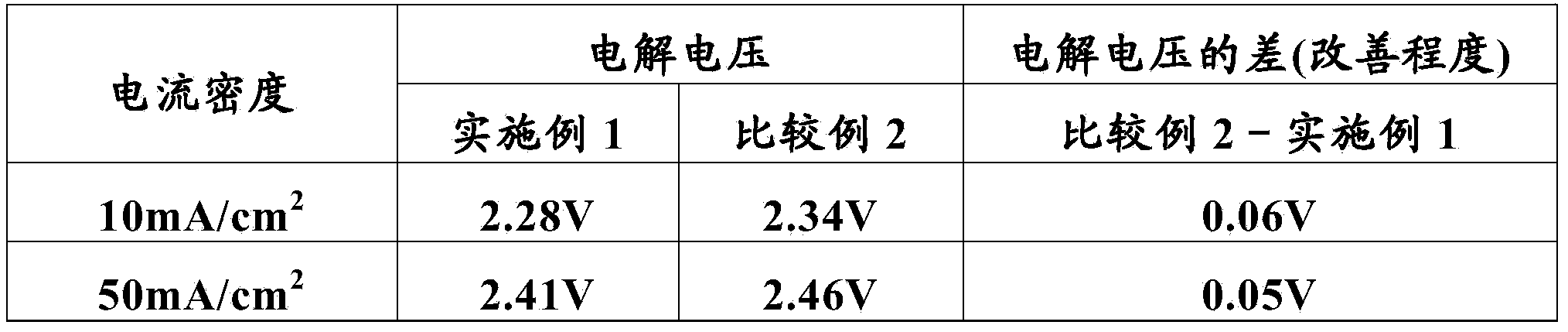
[0085] A commercially available titanium plate (5 cm in length, 1 cm in width, and 1 mm in thickness) was immersed in a 10% oxalic acid solution at 90° C. for 60 minutes for etching treatment, washed with water, and dried. Then, to butanol containing 6vol% concentrated hydrochloric acid (n-C 4 h 9 OH) solution was added ruthenium trichloride trihydrate (RuCl 3 ·3H 2 O) and tantalum pentachloride (TaCl 5 ), the molar ratio of ruthenium and tantalum is 30:70, and the sum of ruthenium and tantalum is 50 g / L in terms of metal, thereby making a coating liquid. This coating solution was applied on the dried titanium plate, dried at 120° C. for 10 minutes, and then thermally decomposed in an electric furnace kept at 260° C. for 20 minutes. This coating, drying, and thermal decomposition were repeated five times in total to form a catalyst layer on a titanium plate as a conductive substrate, thereby producing the anode for electrolytic extraction of Example 1.
[0086] The electr...
Embodiment 2
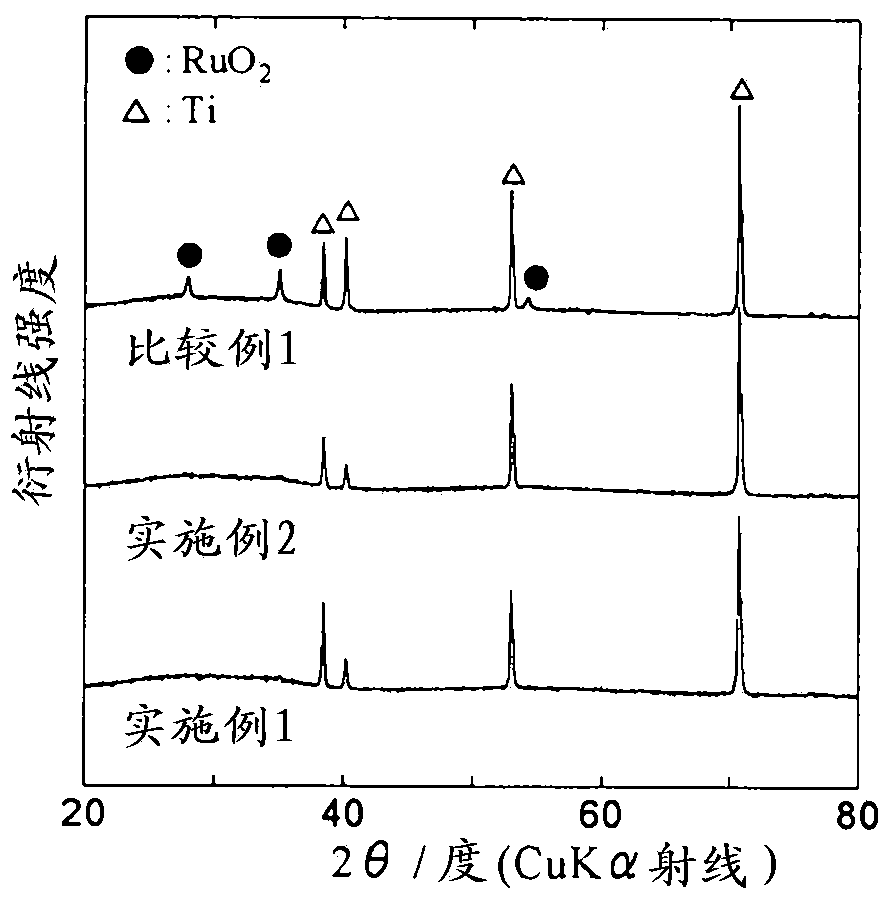
[0089] The anode for electrowinning in Example 2 was produced by the same method as in Example 1, except that the thermal decomposition temperature at the time of forming the catalyst layer was changed from 260°C to 280°C. The electrolytic extraction anode of embodiment 2 is carried out structural analysis by X-ray diffraction method, and the result is: as figure 1 shown, not seen with RuO 2 Comparable diffraction peaks are also not seen with Ta 2 o 5comparable diffraction peaks. In addition, although the diffraction peak of Ti can be seen, it is caused by the titanium plate. That is, in the anode for electrowinning in Example 2, a catalyst layer containing amorphous ruthenium oxide and amorphous tantalum oxide was formed on the titanium plate.
[0090] Preparation of 0.80mol / L ZnSO 4 and 2.0 mol / L sulfuric acid, and a zinc plate (2 cm x 2 cm) was immersed in the electrolyte as a cathode. In addition, the anode for electrolytic extraction of the above-mentioned Example 2...
Embodiment 3
[0112] The electrolytic solution of embodiment 1 becomes by the CuSO of 0.60mol / L 4 And the electrolytic solution that the sulfuric acid of 0.90mol / L constitutes, other conditions are identical with embodiment 1, carry out the electrolytic extraction of copper, measure the terminal voltage (electrolytic voltage) between the anode-cathode of electrolytic extraction simultaneously.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com