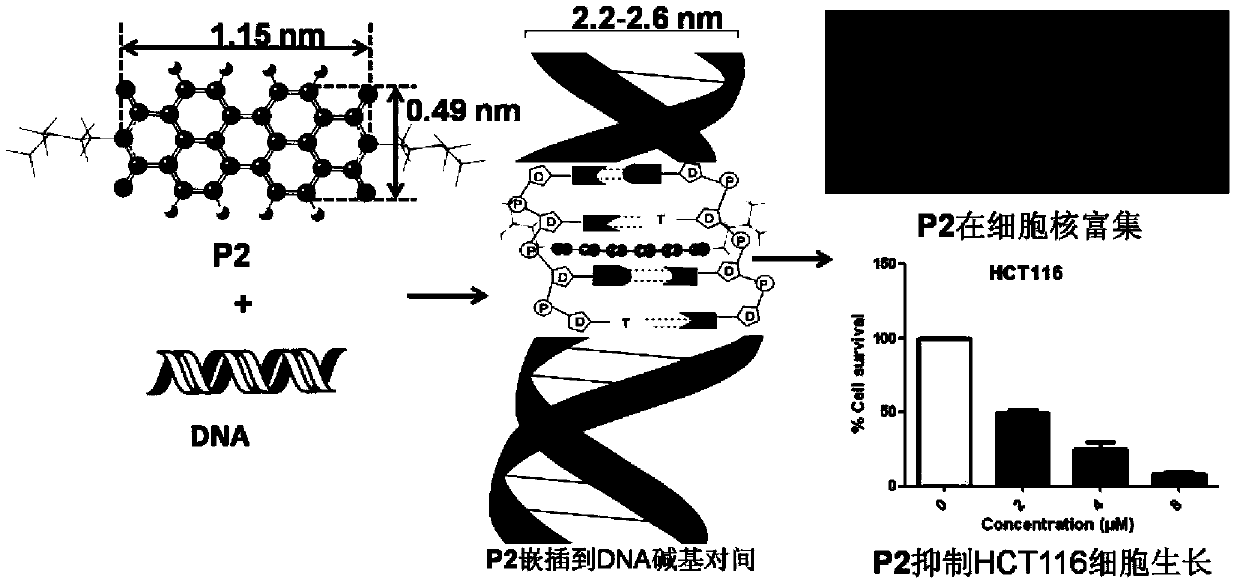
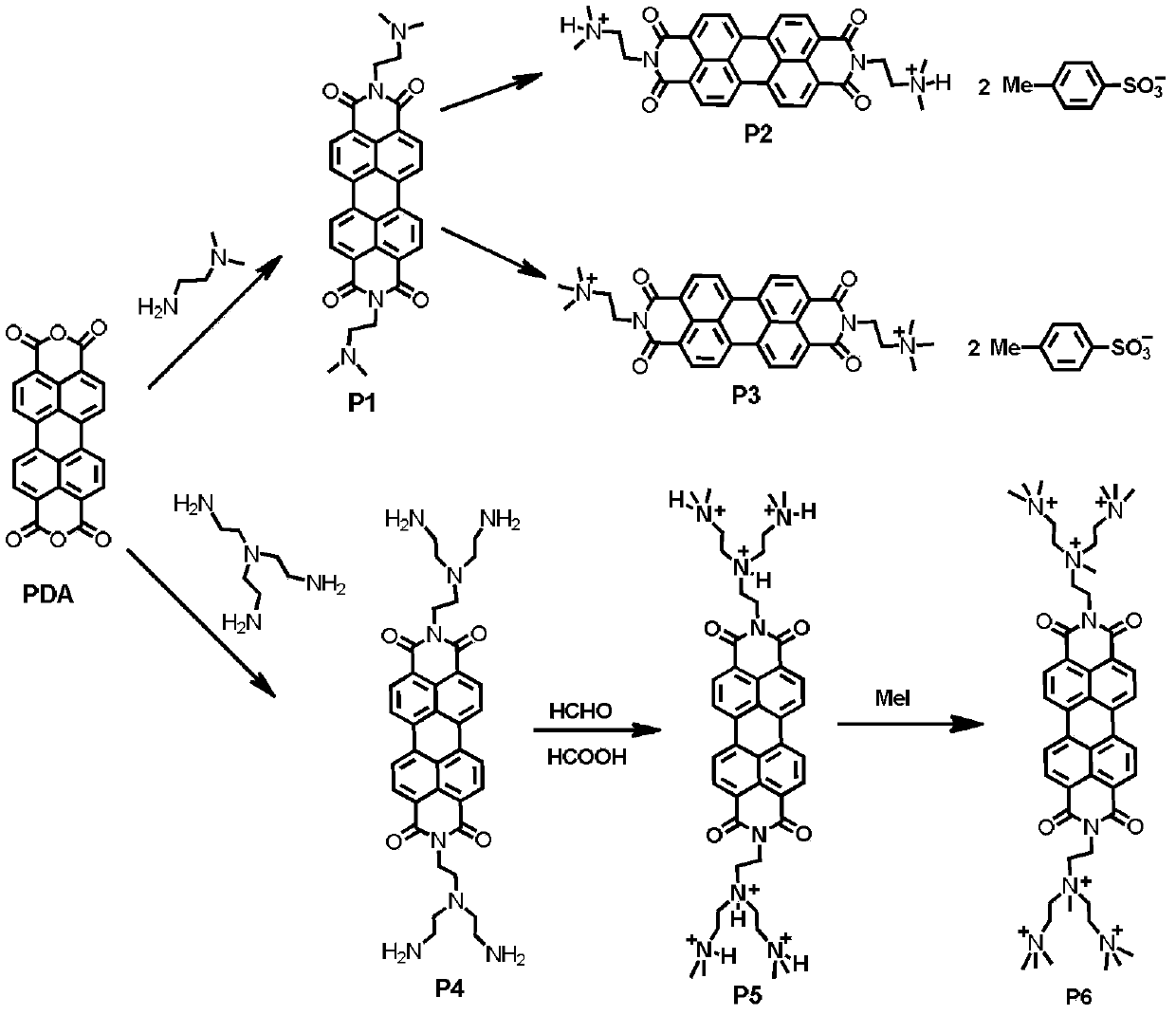
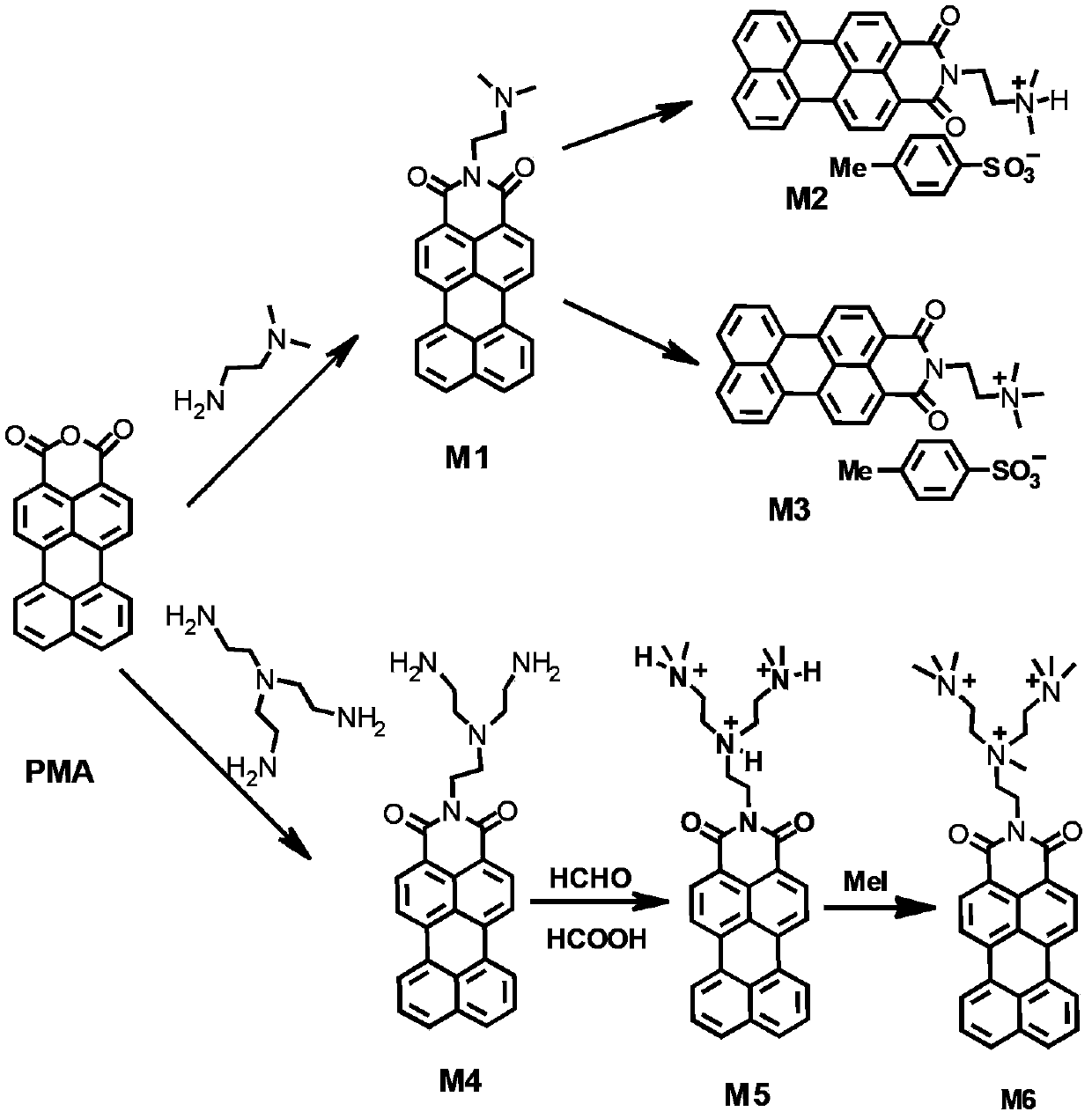
Water-soluble perylene imide compounds, usage as DNA intercalator, and applications thereof in growth inhibition of cancer cells
An amine compound, perylene imide technology, applied in the field of biopharmaceutical chemical synthesis, can solve problems such as research on anti-tumor properties, and achieve the effects of good practical application value, good water solubility, and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1) Suspend 0.7846g perylene anhydride (2.0mmol) and 0.34mL N,N-dimethylethylenediamine (6.0mmol) in 4mL water and stir evenly, then heat up to 100°C and reflux for 12h. After the temperature drops to room temperature, use Wash with 1wt% KOH aqueous solution and water, obtain red solid product P1 after vacuum drying, yield rate is 75%;
[0066] 1 H-NMR (400MHz, CF 3 COOD), δppm: 8.77(d, J=12.3Hz, 8H), 4.77(s, 4H), 3.75(s, 4H), 3.19(s, 12H). 13 C-NMR (100MHz, CF 3 COOD), δppm: 168.67, 138.82, 135.64, 131.77, 128.84, 126.85, 123.97, 60.73, 46.42, 38.74. MS (MALDI-TOF, m / z): CalcdforC 32 h 28 N 4 o 4 ,532.6;Found,533.6(M+H + ).
[0067] 2) 0.32g P1 (0.6mmol) and 1.02g p-toluenesulfonic acid (5.4mmol) were reacted in 3.5mL deionized aqueous solution at 50°C for 4h and then filtered. The precipitate was washed, centrifuged and dried to obtain the red solid product P2 with a yield of 85%.
[0068] 1 H-NMR (400MHz, CF 3 COOD), δppm:8.76(s,8H),7.77(s,13 C-NMR (100MHz...
Embodiment 2
[0077] 1) Add 0.81g perylene anhydride (2.1mmol) and 15mL tris(2-aminoethyl)amine (100.5mmol) into the reflux reaction flask, in which tris(2-aminoethyl)amine is also the reaction solvent; Heat to 100°C for 28 hours under atmosphere, then raise the temperature to 170°C and continue to react for 4 hours. After the temperature drops to room temperature, add a mixed solvent of ethanol and ether with a volume ratio of 1:3, filter, and then wash with toluene and ether The solid product was dried to obtain the red solid product P4 with a yield of 90%.
[0078] 1 H-NMR (400MHz, CF 3 COOD), δppm: 8.82-8.98(m,8H), 4.93(m,4H), 4.40(m,4H), 4.04-4.21(m,16H).UV-Vis(H 2 O,c=10 -5 M):λ max =498nm;Emission(H 2 O,λ ex =498nm):λ max =545nm.MS(MALDI-TOF,m / z):calcdforC 36 h 40 N 8 o 4 :648.75.Found:648.3.
[0079] 2) Add 138mg of P4 to a mixed solution consisting of 0.64mL85wt% formic acid solution and 0.44mL30wt% formaldehyde solution, stir and react at room temperature under nitroge...
Embodiment 3
[0087] (1) Add 0.644g of perylene monoanhydride (2.0mmol) and 0.34mL of N,N-dimethylethylenediamine (6.0mmol) into 3mL of water and stir evenly, then heat up to 110°C, reflux for 15h, after cooling down to room temperature, use Washed with 1wt% KOH aqueous solution and water, and dried in vacuo to obtain dark red solid product M1 with a yield of 79%;
[0088] (2) React 0.196g M1 (0.5mmol) with 0.51g p-toluenesulfonic acid (2.7mmol) in 2mL deionized aqueous solution at 55°C for 5h, then filter, remove the solvent by rotary evaporation, wash with ether precipitation, centrifuge, After drying, red solid product M2 was obtained with a yield of 88%;
[0089] M1 (0.196g, 0.5mmol) was reacted with 1.25mL methyl p-toluenesulfonate (2.7mmol) at 53°C for 19h, and after the temperature dropped to room temperature, filtered, the filtrate was concentrated by rotary evaporation, and then carried out with diethyl ether. The precipitate was washed, centrifuged and dried to obtain the red sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com