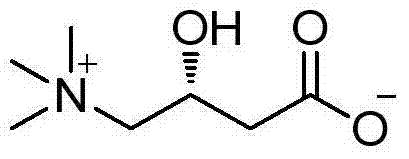
Preparation method for high-optical-purity L-carnitine
An optical and L-carnitine technology, applied in chemical instruments and methods, preparation of organic compounds, cyanide reaction preparation, etc., can solve the problems of cumbersome operation, increased process cost, and inability to obtain high optical pure L-carnitine products, etc. Achieve the effect of low cost, low emission, and suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
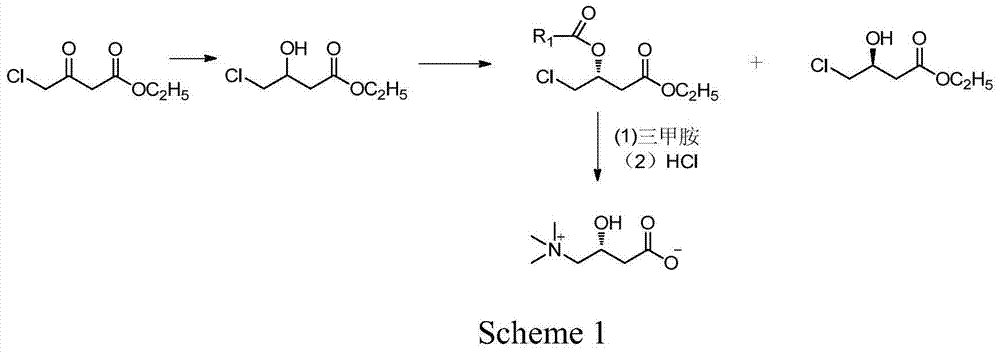
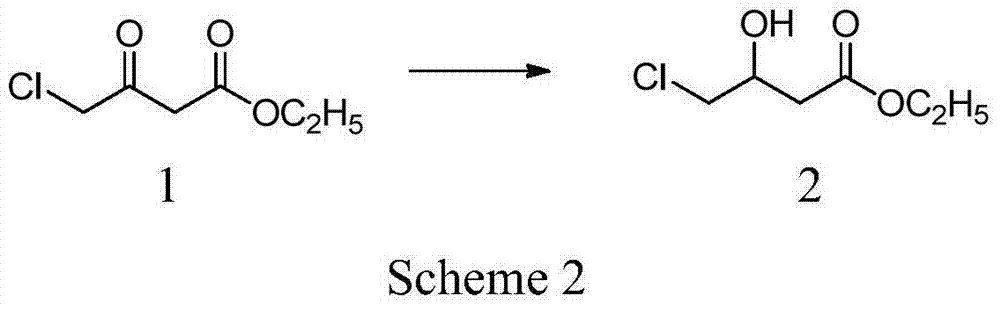
[0041] The preparation of embodiment 14-chloro-hydroxybutyric acid ethyl ester
[0042] At 40°C, 45g (1.19mol) of sodium borohydride was dispersed into 500ml of dried tetrahydrofuran, and stirred for 1h. Dissolve 165g (1mol) of ethyl 4-chloro-acetoacetate in 100ml of tetrahydrofuran and add dropwise to the above sodium borohydride dispersion for about 1 hour. After the dropwise addition, keep stirring for 1 hour. After cooling to room temperature, 200 ml of 5% hydrochloric acid solution was added, and tetrahydrofuran was distilled under reduced pressure. The residue was extracted three times with 300 ml of ethyl acetate, and the combined organic layers were washed with saturated ammonium chloride solution until neutral. Add anhydrous magnesium sulfate for drying, remove the desiccant, and distill ethyl acetate under reduced pressure to obtain 150 g of oily liquid ethyl 4-chloro-hydroxybutyrate with a content of 98% (GC) and a yield of 90%.
Embodiment 2
[0043] The preparation of embodiment 2 (R)-4-chloro-hydroxybutyric acid ethyl ester compound
[0044] 16.7g (100mmol) of ethyl 4-chloro-hydroxybutyrate and 8.3g (80mmol) of methyl methoxyacetate prepared in Example 1 were dissolved in 400ml of methyl tert-butyl ether, and then 7.0g of Novozym435 was added, At room temperature, the reaction was shaken for 7h. After the reaction, the immobilized enzyme was filtered off, and the filtrate was evaporated to dryness to obtain an oily residue. The residue was passed through a silica gel column and eluted with ethyl acetate. First, the ethyl acetate solution of (R)-4-chloro-hydroxybutyric acid ethyl ester was collected, and then (S)-4-chloro-hydroxybutyrate was collected. The ethyl acetate solution of ethyl acetate was evaporated to dryness under reduced pressure respectively to obtain 11.8 g of (R)-4-chloro-hydroxybutyric acid ethyl ester esterified product, content 99%, yield 48.7%, (S)-4-chloro- -Ethyl hydroxybutyrate 8.1g, conte...
Embodiment 3
[0045] The preparation of embodiment 3L-carnitine
[0046] Add 11.8g (49mmol) of the esterified product obtained in Example 2, 35g (154mmol) of trimethylamine aqueous solution (26%) and 35mL of ethanol into the reaction flask, and stir the reaction mixture at about 80°C for 5h, then cool to room temperature. Ethanol and unreacted trimethylamine were removed by pressure steaming. Add 50ml of 10% hydrochloric acid to the residue, reflux for 4h, evaporate 50ml of water under reduced pressure, add 55mL of isopropanol to the obtained residue for recrystallization, filter to obtain a white solid. The white solid was dissolved in water and desalted with 732-type cation exchange resin to obtain about 6.5 g of L-carnitine with a content of 99% (HPLC), a yield of 82.4%, and an ee value of 99.3%, [α] 2 D =-30 (c=1, H 2 o).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com