A kind of magnetic heavy metal adsorbent with shell-core structure and preparation method thereof
A nuclear structure, heavy metal technology, applied in the direction of alkali metal compounds, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve the problems of large specific surface area, secondary pollution, separation difficulties, etc., achieve high adsorption capacity and reduce treatment The effect of simple cost and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
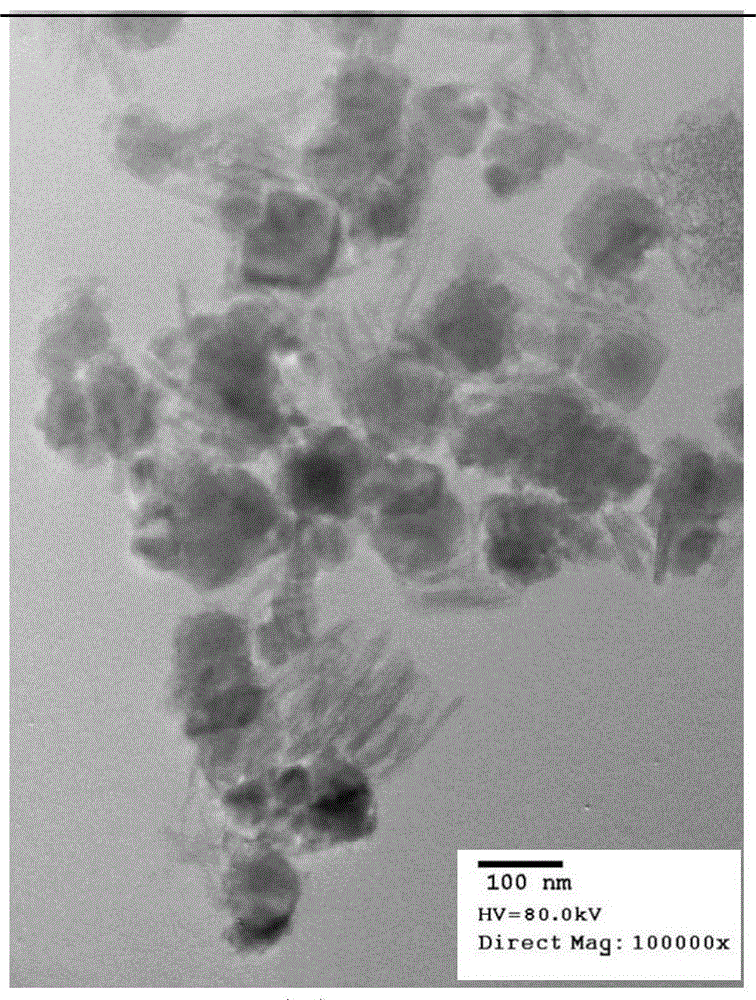
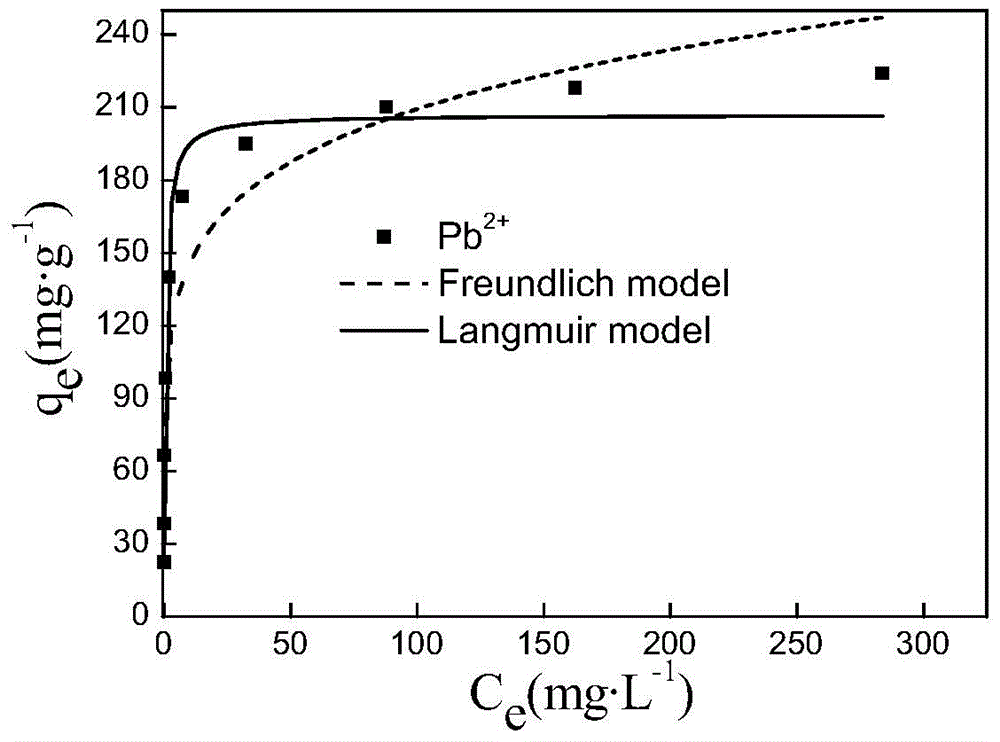
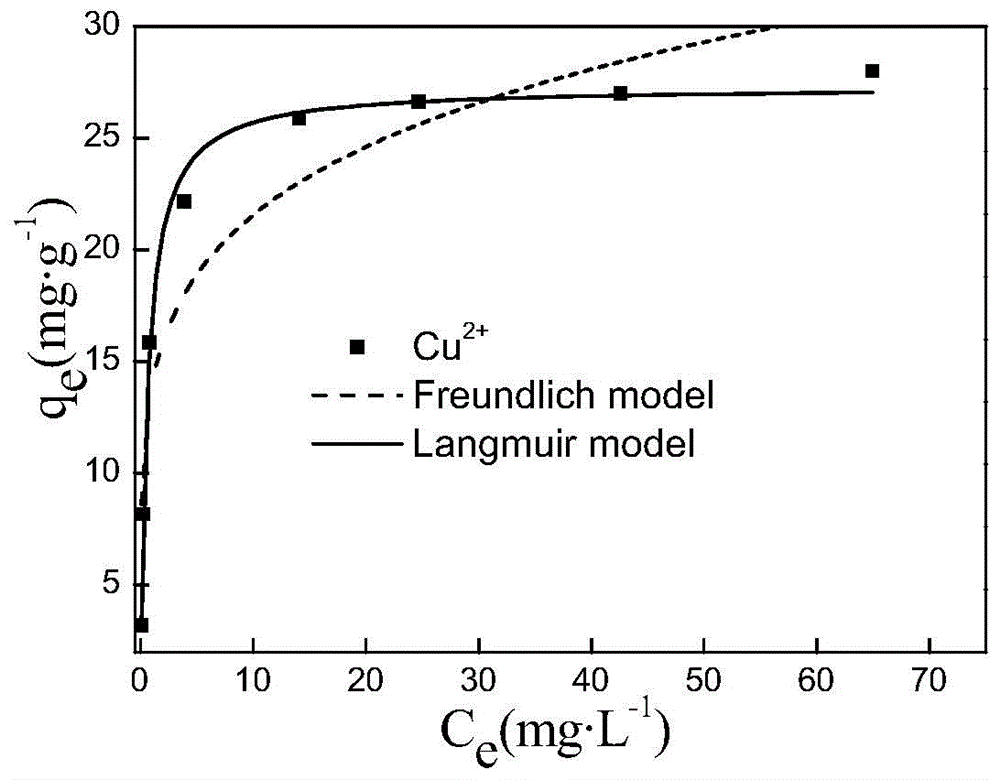
[0030] Use ferric chloride and ferrous chloride as raw materials to prepare Fe 3+ and Fe 2+ Add 500 mL of the mixed solution with a molar ratio of 2:1, slowly add 2 mol / L sodium hydroxide solution into the mixed solution, and stir until the pH of the mixed solution is 11. The mixed solution was reacted at 60°C for 2 hours, aged at room temperature for 30 minutes, solid-liquid separation was carried out under the action of a magnetic field, and washed with deionized water for 3-4 times to obtain the core magnetic nano-iron-manganese composite Mn x Fe 3-x o 4 (x=0). Weigh 1.0 g of the prepared core Mn x Fe 3-x o 4 (x=0), mixed with 100mL of 2% PEG solution, mixed with 120mL of 0.036mol / L manganese chloride solution, heated to 60°C, and slowly added dropwise with 80mL of 0.036mol / L potassium permanganate solution , stir vigorously until the addition is complete, react at room temperature, the manganese dioxide obtained from the reaction is directly coated on the surface of...
Embodiment 2
[0032] Ferric sulfate, ferrous sulfate and manganese sulfate are used as raw materials to prepare Fe 3+ , Fe 2+ with Mn 2+ Add 500 mL of a mixed solution with a molar ratio of 2:0.5:0.5, slowly add 2 mol / L potassium hydroxide solution to the mixed solution, and stir until the pH of the mixed solution is 11. The mixed solution was reacted at room temperature for 2 hours, aged at room temperature for 30 minutes, solid-liquid separation was carried out under the action of a magnetic field, and washed 3-4 times with deionized water to obtain the core magnetic nano-iron-manganese composite Mn x Fe 3-x o 4 (x=0.5). Weigh 1.0 g of the prepared core Mn x Fe 3-x o 4 (x=0.5), mixed with 50mL5% PEG solution, mixed with 120mL0.036mol / L manganese chloride solution, heated to 60°C, then slowly added dropwise 80mL0.036mol / L sodium permanganate solution , stir vigorously until the addition is complete, react at room temperature, the manganese dioxide obtained from the reaction is dire...
Embodiment 3
[0034] Using ferric nitrate and manganese nitrate as raw materials to prepare Fe 3+ with Mn 2+ Add 500 mL of the mixed solution with a molar ratio of 2:1, slowly add 2 mol / L sodium hydroxide solution into the mixed solution, and stir until the pH of the mixed solution is 11. The mixed solution was reacted at 60°C for 2 hours, aged at room temperature for 30 minutes, solid-liquid separation was carried out under the action of a magnetic field, and washed 3-4 times with deionized water to obtain the core magnetic nano-iron-manganese composite Mn x Fe 3-x o 4 (x=1). Weigh 1.0 g of the prepared core Mn x Fe 3-x o 4(x=1), mixed with 20mL10% PEG solution, mixed with 200mL0.036mol / L manganese nitrate solution, heated to 60°C, then slowly added dropwise 40mL0.036mol / L sodium permanganate solution, Stir vigorously until the dropwise addition is complete, react at room temperature, and the manganese trioxide obtained from the reaction is directly coated on the surface of the inne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com