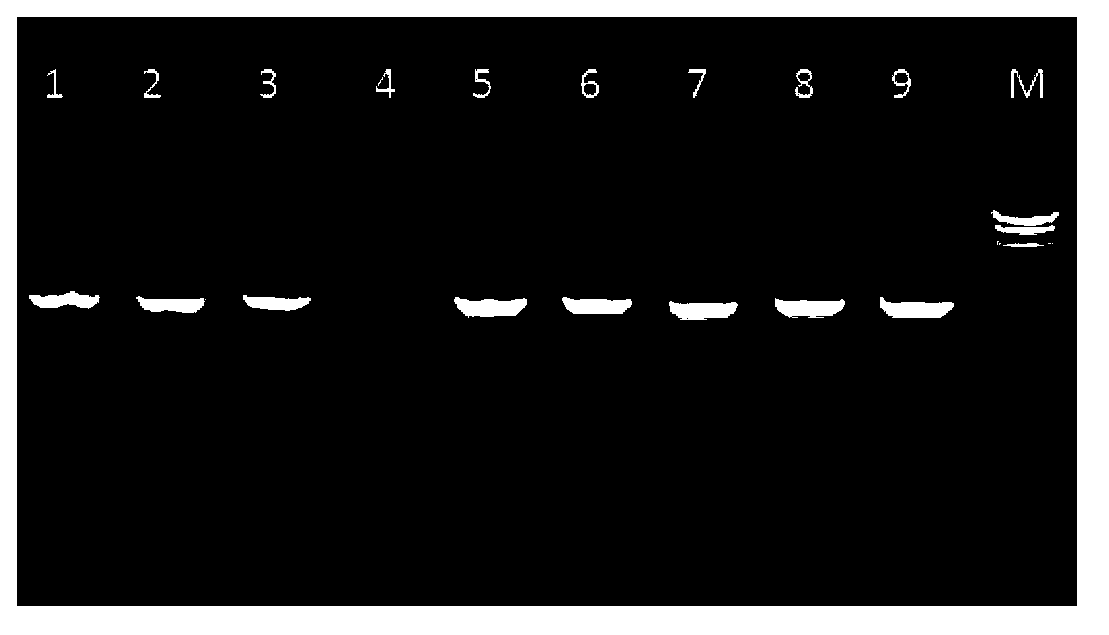
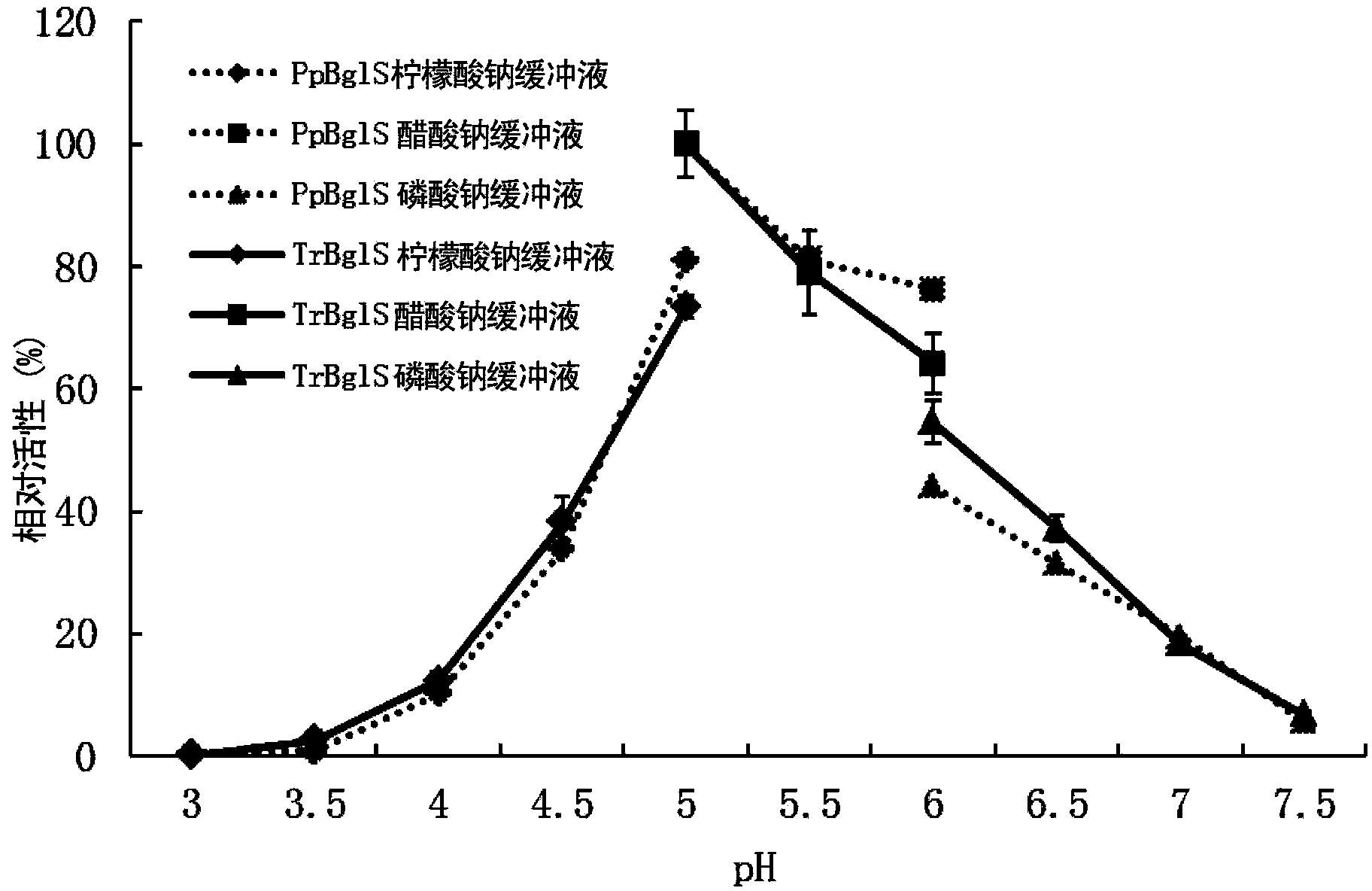
Novel beta-glucosidase, coding gene thereof and applications thereof
A technology of application and glycosidic bond, applied in the direction of glycosylase, genetic engineering, plant genetic improvement, etc., can solve the problems of reducing industrial production cost and insufficient yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Embodiment 1, the isolation of β-glucosidase and its coding gene
[0122] At 37°C, culture Aspergillus terreus (NIH2624, purchased from the American Type Culture Collection) for 2 days with cellulose-containing Chapei medium, extract RNA, and then reverse-transcribe it into cDNA, search through the Aspergillus terreus genome database bgls gene number Gene: ATEG_07931 (http: / / www.cadre-genomes.org.uk / Aspergillus_terreus), using cDNA as a template, using polymerase chain reaction to separate and synthesize double-stranded DNA, the isolated gene has SEQ ID NO: The nucleotide sequence of 1 is the bgls coding sequence. From the 1st to 2211th nucleotides of the 5' end of SEQ ID NO:1 is the open reading frame (Open Reading Frame, ORF) of bgls, from the 1st-3rd nucleotides of the 5' end of SEQ ID NO:1 It is the initiation codon ATG of the bgls gene, and the 2209th to 2211th nucleotides from the 5' end of SEQ ID NO: 1 are the termination codon TGA of the bgls gene.
[0123]The...
Embodiment 2
[0125] Embodiment 2, secretory expression of bgls in host Trichoderma reesei
[0126] 1. Host modification for optimized expression of bgls
[0127] Site-directed mutation was performed on the cbh1 promoter (SEQ ID NO: 13) of Trichoderma strain RC30-8 (see Microbial Cell Factories, 2012, 11:21). There are three carbon metabolism repressor (CCR) protein Cre1 binding sites (5'-SYGGRG-3' or its complementary sequence) in the cbh1 promoter, resulting in insufficient expression intensity under glucose repression conditions. In the first round of mutation, the Cre1 binding site at -724 was transformed into the binding site of the transcriptional activator Ace2 (5'-GGCTAA-3') by point mutation; in the second round of mutation, -698 and Both CRE1 binding sites at -690 were mutated into the binding site of Hap protein complex (5'-CCAAT-3'), and the mutant promoter pchb1m2 (SEQ ID NO: 14) was obtained. Taking eGFP as the reporter gene, it was found that after the promoter was mutated ...
Embodiment 3
[0139] Example 3, secretory expression of bgls in host Pichia pastoris
[0140] 1. Construction of recombinant expression vector in host Pichia pastoris
[0141] The gene isolated from the above by PCR is used as a template to clone the β-glucosidase ORF coding gene (except for the signal peptide coding sequence), and the forward primer used is: 5'AT ATCGAT TCTGACCACCTGGGACGCGGC3'(SEQ ID NO:6), Cla I recognition site added to its 5' end: ATCGAT; reverse primer is 5'GC TCTAGA GGAACAGTAAAAGACCCATCC3' (SEQ ID NO: 7), with an Xba I recognition site added to its 5' end: TCTAGA.
[0142] After the PCR product was purified, it was digested with Cla I and Xba I, and the digested DNA fragment was recovered using the Axygen PCR Product Column Recovery Kit. The DNA fragment and the recovered vector pPICZαC (purchased from Invitrogen) , were ligated overnight at 16°C with T4 DNA ligase to obtain the recombinant expression vector pPICZαC-ppbgls. The C-terminus of the expression produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com