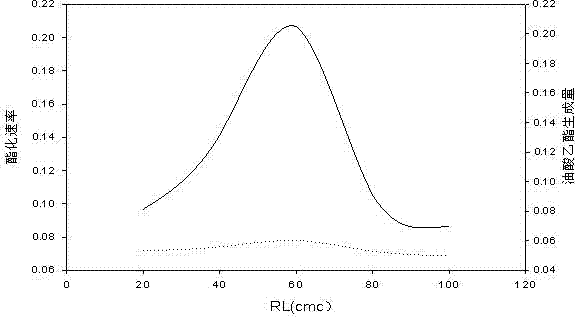
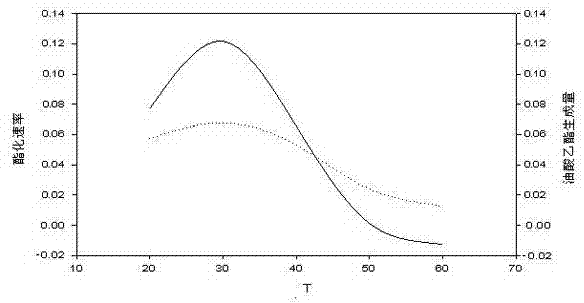
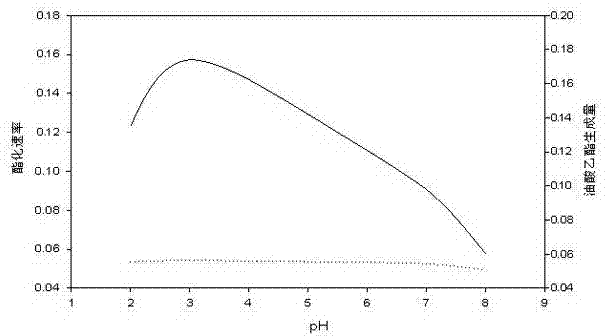
Application of reverse micelle system in catalysis of lignin peroxidase for esterification reaction of oleic acid
A technology of peroxidase and reverse micelles, applied in the field of treatment of oleic acid pollution, to achieve the effect of mild production conditions, mild reaction conditions, and simple production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Use a pipette to take 500ml each of isooctane and n-hexanol into a beaker, take 4.1448g of rhamnolipid in a 1L volumetric flask to volume, and ultrasonically dissolve until the solution is clear to obtain a 100CMC RL-isooctane-n-hexanol solution.
[0020] 1.5 mg of lignin peroxidase was dissolved in 15 ml of disodium hydrogen phosphate-citric acid buffer solution with pH=3 to prepare a crude enzyme solution. Dissolve 1.782ml of crude enzyme solution in 10ml of the above RL-isooctane-n-hexanol solution to form a reverse micellar enzyme system, wherein the molar ratio (Wo) of water / rhamnolipid is 30. Add 40mmol oleic acid and 10mmol ethanol to the reverse micelles enzyme system, put it into a water bath shaking box, the temperature is 25°C, the rotation speed is 150rpm, and the reaction time is one hour.
[0021] Every 10 minutes, take 1ml of the reaction product, add 1ml of copper acetate (containing pyridine with a pH of 6.1) and 5ml of benzene, and centrifuge in a cent...
Embodiment 2
[0023] Use a pipette to take 500ml each of isooctane and n-hexanol into a beaker, take 2.1448g of rhamnolipid in a 1L volumetric flask to volume, ultrasonically dissolve until the solution is clear, and obtain a 20CMC RL-isooctane-n-hexanol solution.
[0024] Dissolve 5.4 mg of lignin peroxidase enzyme in 50 ml of disodium hydrogen phosphate-citric acid buffer solution with pH=2 to prepare a crude enzyme solution. Dissolve 2.376ml of crude enzyme solution in 10ml of the above-mentioned RL-isoctane-n-hexanol solution to form a reverse micellar enzyme system, wherein the molar ratio (Wo) of water / rhamnolipid is 40, and the Add 60mmol oleic acid and 10mmol ethanol to the mixture, put it into a water bath shaking box, the temperature is 40°C, the rotation speed is 150rpm, and the reaction time is one hour.
[0025] Every 10 minutes, take 1ml of the reaction product, add 1ml of copper acetate (containing pyridine with a pH of 6.1) and 5ml of benzene, and centrifuge in a centrifuge ...
Embodiment 3
[0027] Use a pipette to take 500ml each of isooctane and n-hexanol into a beaker, take 3.2418g of rhamnolipid in a 1L volumetric flask to volume, ultrasonically dissolve until the solution is clear, and obtain a 40CMC RL-isooctane-n-hexanol solution.
[0028] Take 4 mg of lignin peroxidase and dissolve it in 10 ml of disodium hydrogen phosphate-citric acid buffer solution with pH=5 to prepare a crude enzyme solution. Dissolve 1.934ml of crude enzyme solution in 10ml of the above RL-isooctane-n-hexanol solution to form a reverse micellar enzyme system, wherein the molar ratio (Wo) of water / rhamnolipid is 34. Add 57mmol oleic acid and 14mmol n-propanol to the reverse micellar enzyme system, put it into a water bath shaking box, the temperature is 32°C, the rotation speed is 150rpm, and the reaction time is 2 hours.
[0029] Every 10 minutes, take 1ml of the reaction product, add 1ml of copper acetate (containing pyridine with a pH of 6.1) and 5ml of benzene, and centrifuge in a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com