Method for extracting potassium nitrate from circulating mother liquor for producing aluminum oxide through Bayer process
A technology of circulating mother liquor and Bayer process, which is applied in the direction of preparation of alkali metal nitrates, etc., can solve the problems that cannot meet the needs of the Bayer process process and the process is complicated, and achieve the effects of strong product market competitiveness, low production cost, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
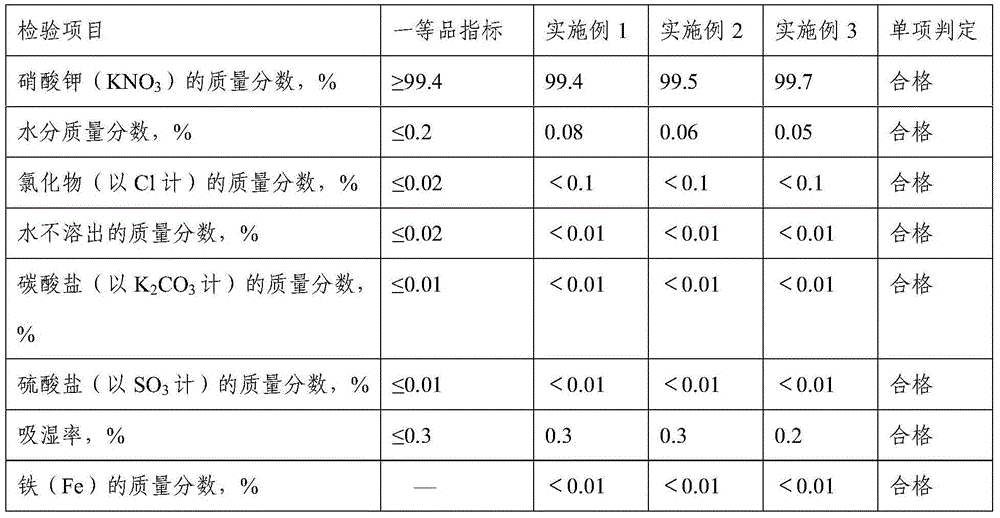
Embodiment 1
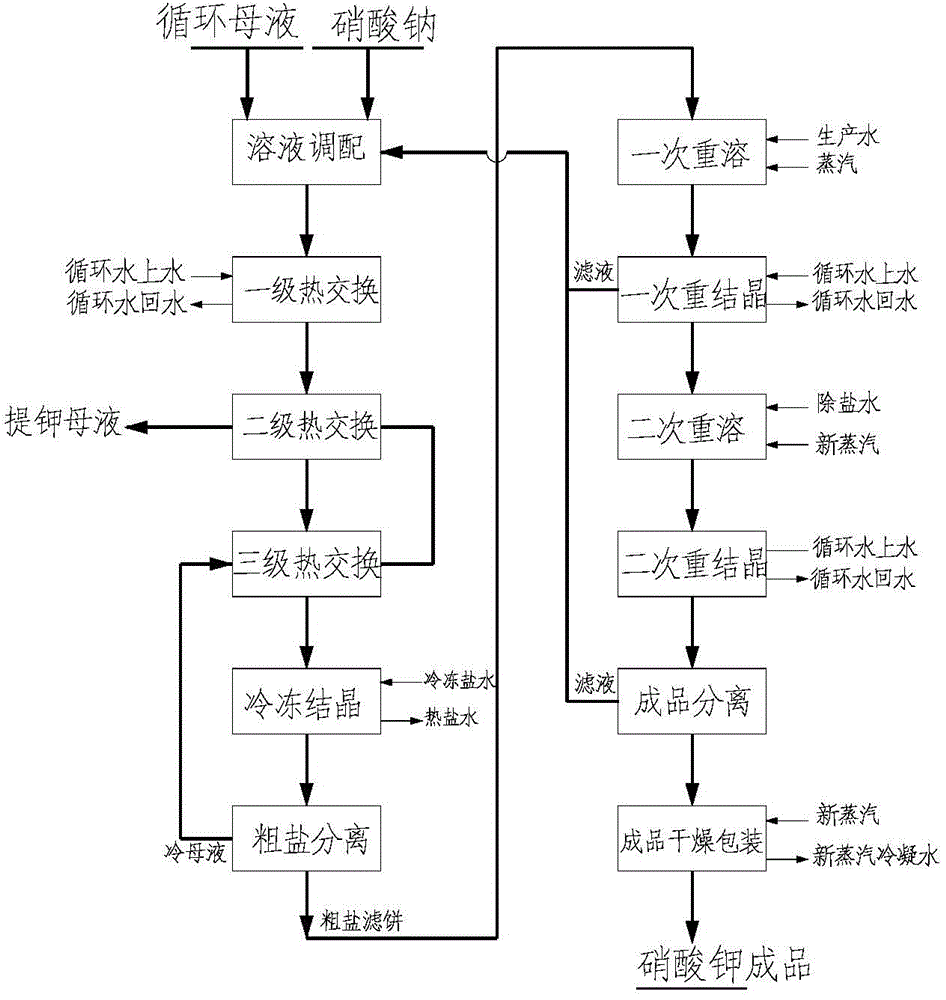
[0039] Process such as figure 1 .
[0040]1. Use the Bayer method to circulate the mother liquor (or the forced mother liquor of the evaporator) to dissolve sodium nitrate. In the initial stage of the system, the purified mother liquor after centrifugation has not yet been produced, and the sodium nitrate is 70%K 2 O crystallization ratio is added in equimolar ratio, and sodium nitrate is added to make K in the mixed solution + with NO 3- The molar ratio is 1:0.7. The caustic alkali concentration in the mixed solution is 240g / L.
[0041] 2. The mother liquor is prepared for three-stage cooling, and the cooling equipment is a wide channel plate heat exchanger. Considering the full use of heat, circulating water is used for the first stage of cooling, crystallization mother liquor is used for the second stage of cooling, and frozen brine is used for the third stage of cooling. After the third cooling, the temperature is controlled at -20 °C. After the second cooling, the cr...
Embodiment 2
[0047] 1. Dissolve sodium nitrate with the Bayer process circulation mother liquor, and the solution mixes with the purified mother liquor returned in step 3 and step 4, so that K in the mixed liquor + with NO 3- The molar ratio is 1:0.8; the evaporator is added to force the mother liquor to control the caustic alkali concentration in the mixed solution to not be lower than 220g / l.
[0048] 2. The mother liquor is prepared for three-stage cooling, and the cooling equipment is a wide channel plate heat exchanger. After the first stage of cooling, the temperature is controlled at 43°C, after the second stage of cooling, the temperature is controlled at 5°C, after the third stage of cooling, the temperature is controlled at -15°C, and after the second stage of cooling, the temperature of the crystallization mother liquor used as a cooling medium is raised to 30°C The above returns to the alumina refinery.
[0049] 3. After cooling down for the third time, mix the liquid and liq...
Embodiment 3
[0054] 1. Use the Bayer method to circulate the mother liquor to dissolve sodium nitrate, and mix the solution with the purified mother liquor returned from step 3 and step 4 to make NO in the mixed liquor 3- with K + The molar ratio is 1:1; add evaporating force mother liquor to control the caustic alkali concentration in the mixed solution not less than 220g / l.
[0055] 2. The mother liquor is prepared for three-stage cooling, and the cooling equipment is a wide channel plate heat exchanger. After the first stage of cooling, the temperature is controlled at 40°C, and after the second stage of cooling, the temperature is controlled at 0°C. After the third cooling, the temperature is controlled at -15°C. After the second cooling, the crystallization mother liquor used as cooling medium is raised to above 30°C and returned to the alumina plant.
[0056] 3. After the third cooling, the prepared solution was stirred at constant temperature for 1 hour, and the obtained crude pot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com