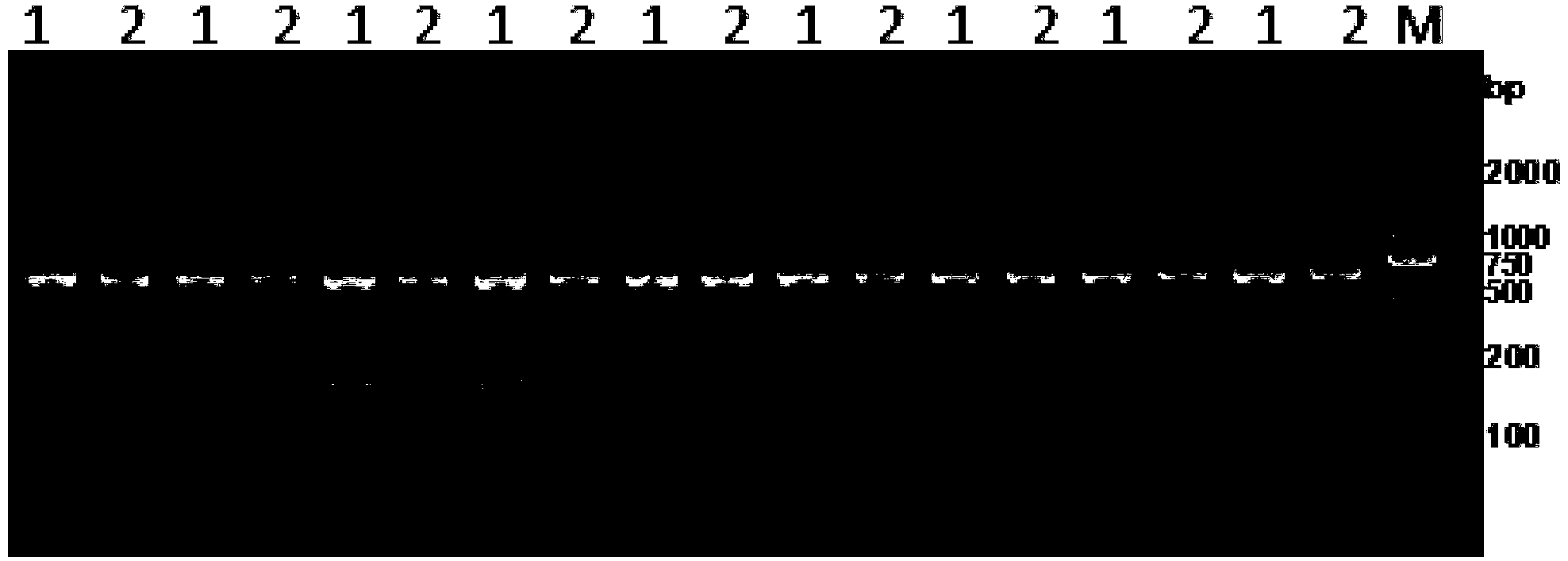Sample conditioning fluid based on denaturation precipitation as well as application thereof and nucleic acid releasing method
A technology for sample treatment liquid and nucleic acid release, which is applied in biochemical equipment and methods, and the determination/inspection of microorganisms. It can solve the problems of complex operation, low recovery efficiency, and high cost, and achieves reduction of pollution sources, guaranteeing stability, and avoiding errors. and the effects of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Technical principle of the present invention
[0035] Polyethylene glycol can induce the aggregation of macromolecules in aqueous solution. Under the action of polyethylene glycol, it can increase the hydrophilicity of the solution and increase the concentration of OH- ions in the solution. The strong alkaline environment provided is conducive to the rapid lysis of cells. And destroy the nucleoprotein structure, and when it is added to the PCR reaction solution, its pH value will be reduced to the normal level, and will not inhibit the amplification.
[0036] High salt solution is conducive to the precipitation of nucleoproteins in cells. Solid-phase chelating resin can integrate multivalent metal ions, especially divalent ions selectively. It has higher metal ion selectivity and stronger binding than ordinary ion exchangers. It can combine many other foreign substances (such as ferrous ions) that may affect the one-step analysis, and remove solid-phase par...
Embodiment 2
[0043] The sample treatment solution of the present invention is composed of (accounting for the mass percentage of the sample treatment solution) 1-15% PEG400, 10-40mmol / L potassium hydroxide (KOH), 0.1-2% surfactant (Teween-20, TritonX-100 ), 1-20% solid phase resin chelex-100, 3-20% PCR enhancer (glycerol, DMSO) and 100-400mmol / L KCL.
[0044] Configuration method: add 1-15% PEG400 and 10-40mmol / L potassium hydroxide (KOH) with sterile water, add 0.1-2% surfactant (Teween-20, TritonX-100) and 100-400mmol / L KCL, then add 3-20% PCR synergist (glycerol, DMSO), and finally add 1-20% solid phase resin chelex-100 and mix well to get the sample treatment solution.
[0045] The prepared sample processing solution and the blood sample are added and mixed according to a certain ratio. The preferred adding ratio of blood sample is 20 μL of blood sample added to 20-500 μL of lysate, vortexed and mixed, and more preferably 20 μL of blood sample is added to 50 -150 μL of the lysate, vor...
Embodiment 3
[0048] A preferred sample treatment solution of the present invention has the following components (accounting for the mass percentage of the sample treatment solution): 3% PEG400, 0.5-1.2% surfactant, 5-15% solid phase resin chelex-100, 5- 8% PCR booster, 150-300mmol / LKCL and 20mmol / LKOH. The sample processing liquid solvent is sterile water.
[0049] The use of the above-mentioned sample treatment liquid of the present invention:
[0050] 1. Take out the sample treatment solution (the reagent is a suspension, shake it well before use to suspend the particles), add 300 μL of the sample treatment solution to the 1.5mL centrifuge tube, and use a pipette from time to time during the process of absorbing the sample treatment solution. Pipette the liquid to make the particles evenly suspended.
[0051] 2. Add 20.0 ul of anticoagulated blood to the sample treatment solution, vortex for 25 sec, and centrifuge briefly to avoid residual droplets on the inner wall of the tube.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com