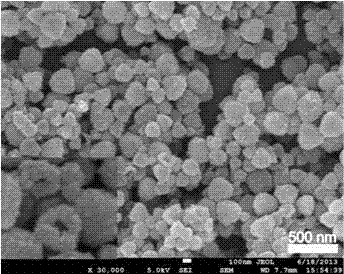
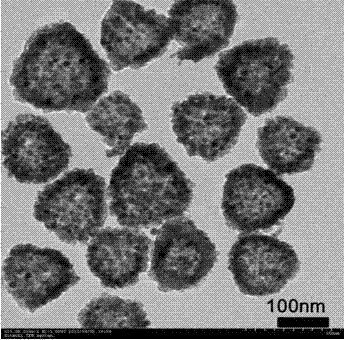
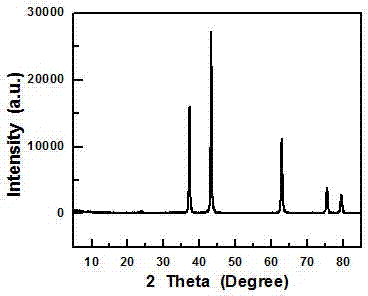
Lithium ion battery made of hollow porous nickel oxide composite material on basis of coating of N-doped carbon layer, and preparation method thereof
A lithium-ion battery, hollow porous technology, applied in the manufacture of electrolyte batteries, battery electrodes, secondary batteries, etc., can solve the problems of poor electronic conductivity, limited electrochemical performance of nickel oxide, battery cycle performance, and low lithium ion diffusion rate. , to achieve the effects of good discharge performance, reduced migration and diffusion rates, and high cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In 20 mL of ethylene glycol, 0.895 g of nickel chloride hexahydrate, 1.23 g of sodium acetate, and 1.2 g of urea were added respectively, and the mixture was magnetically stirred at 20-25 °C for 3-5 h. After mixing evenly, pour it into a stainless steel reactor lined with polytetrafluoroethylene, heat in an oven at 160-200 °C for 18-24 h, and cool to room temperature. After the obtained product was centrifuged and washed with ethanol and deionized water for 6 to 8 times, it was placed in a drying oven at 60 to 100 °C for 12 to 24 h under vacuum to obtain precursor nickel bicarbonate nanoparticles with a particle size of about 200 to 300 nm. Add 3 mg of the precursor to 5 mL of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, and disperse by ultrasonication for 5 to 15 min. Put the dispersion liquid in a stainless steel reaction kettle lined with polytetrafluoroethylene, solvothermally treat it at 160-200 °C for 3-5 h, cool to room temperature, and centrifuge...
Embodiment 2
[0045] In 20 mL of ethylene glycol, 1.0 g of nickel chloride hexahydrate, 1.23 g of sodium acetate, and 0.8 g of urea were added respectively, and the mixture was magnetically stirred at 20-25 °C for 3-5 h. After mixing evenly, pour it into a stainless steel reactor lined with polytetrafluoroethylene, heat at 160-200 °C for 18-24 h, and cool to room temperature. After the obtained product was centrifuged and washed with ethanol and deionized water for 6 to 8 times, it was placed in a drying oven at 60 to 100 °C for 12 to 24 h under vacuum to obtain precursor nickel bicarbonate nanoparticles with a particle size of about 200 to 300 nm. Add 80 mg of this precursor to 10 ml of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, and ultrasonically disperse for 5-15 min. Place the dispersed solution in a stainless steel reaction kettle lined with polytetrafluoroethylene, treat it with solvothermal method at 160-200 °C for 3-5 h, cool to room temperature, and centrifuge the...
Embodiment 3
[0048] The charge-discharge curve ( Figure 6 ) and the charge-discharge curve at a current density of 0.1C ( Figure 5 ). The test results show that under the same conditions of other operations, the charging current density is changed to 0.3 C, and the discharge specific capacity of the obtained lithium-ion battery is about 587.3 mAh / g ( Figure 6 curve b); the charge current density is changed to 1.0 C, and the discharge specific capacity is about 479.4 mAh / g ( Figure 6 curve c); the charging current density is changed to 10 C, and the discharge specific capacity of the lithium-ion battery is about 347.6 mAh / g ( Figure 6 Curve d). The cycle performance test of discharge shows that the lithium-ion battery cycle performance is good ( Figure 5 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com