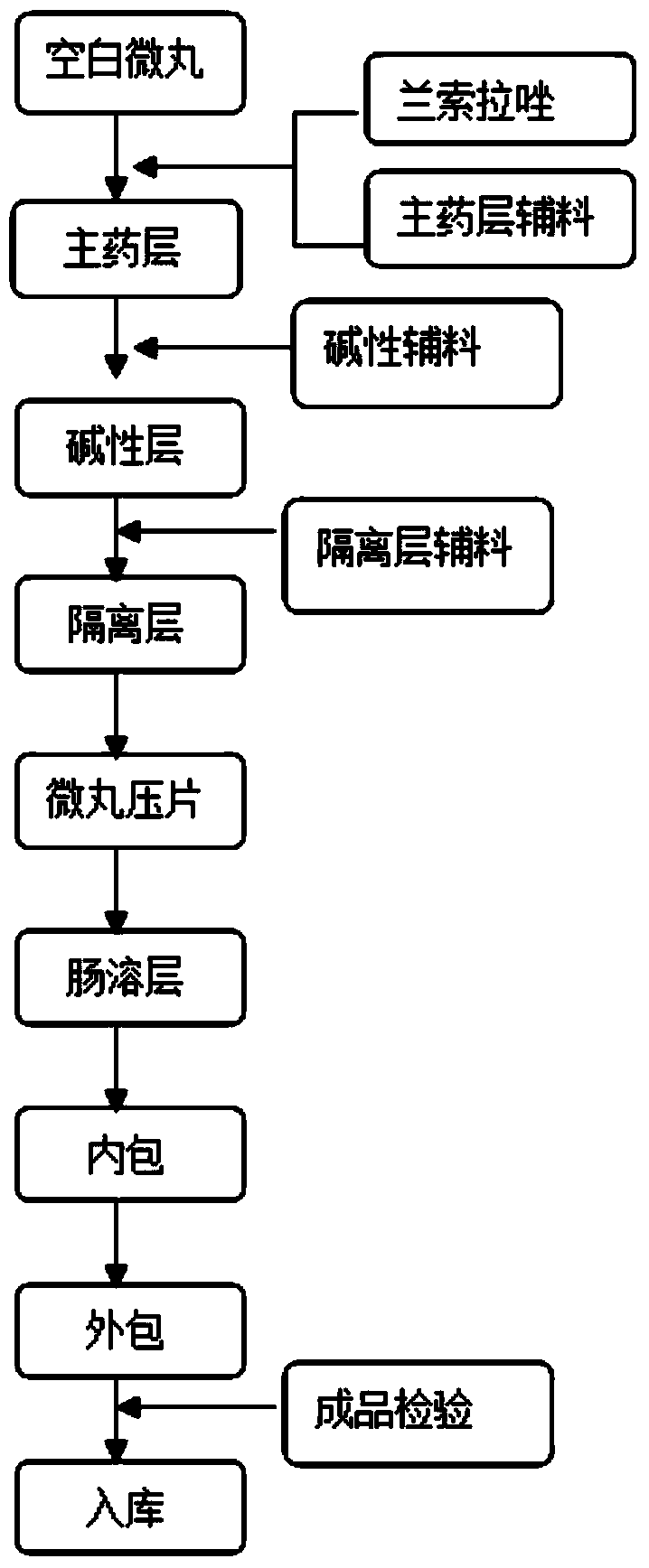
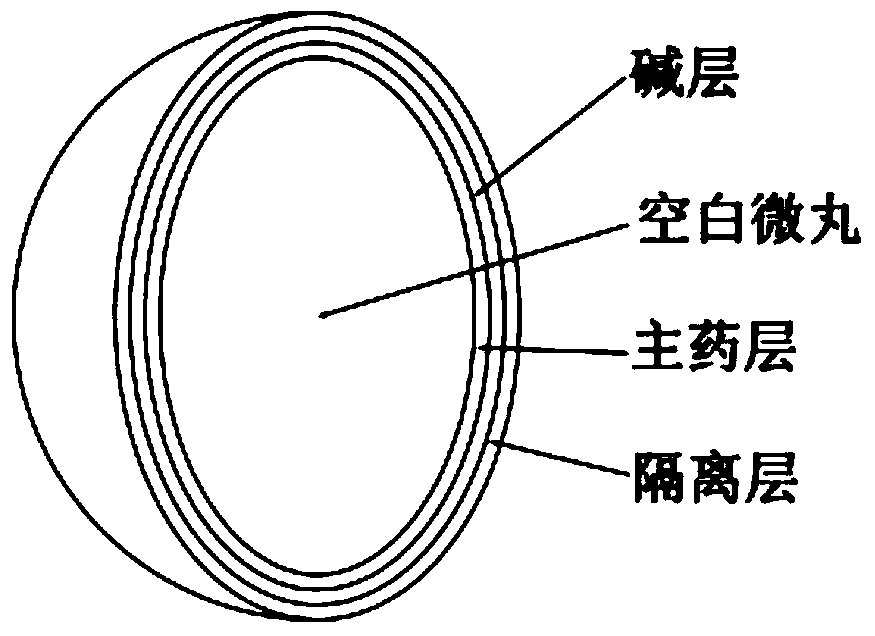
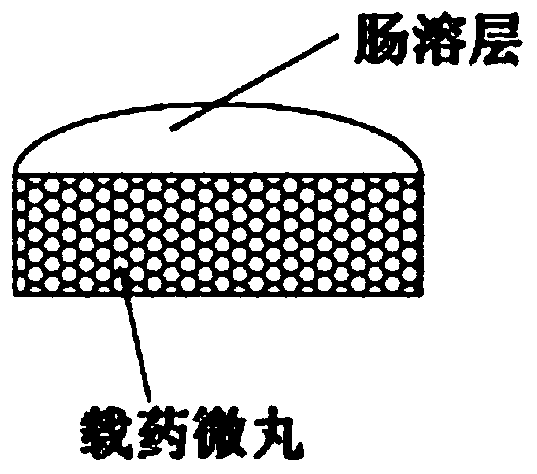
Stable, uniform and efficient lansoprazole enteric-coated tablets and preparation method thereof
A technology of lansoprazole enteric and enteric-coated tablets, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulas, etc., can solve the problem of destroying lansoprazole, poor uniformity, Release rate is not high, to achieve the effect of improving release rate and bioavailability, improving content uniformity, and good quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Prescription: (according to 1000 tablets)
[0044] Blank pellets (sucrose type): 200g
[0045] Main drug layer:
[0046] Lansoprazole 30g
[0047] Hypromellose 10g
[0048] Tween-80 2g
[0049] Alkaline layer:
[0050] Hypromellose 5g
[0051] Basic magnesium carbonate 5g
[0052] Isolation layer:
[0053]
[0054] Enteric layer:
[0055] Enteric coating solution 125g
[0056] Triethyl citrate 4g
[0058] Preparation:
[0059] (1) Preparation of drug-loaded pellets: Take the prescribed amount of sodium carboxypropylmethylcellulose, add an appropriate amount of purified water and stir to dissolve, add the prescribed amount of Tween-80, stir to dissolve completely, add water to 200mL, and then add the prescription Amount of lansoprazole raw material, stirred to obtain a uniform suspension solution.
[0060] Weigh the prescribed amount of blank pellets, place them in a fluidized bed coating machine, ad...
experiment example 1
[0067] This experimental example relates to the content uniformity inspection of the lansoprazole enteric-coated tablets prepared in Example 1 of the present invention.
[0068] According to the content uniformity inspection method stipulated in the Chinese Pharmacopoeia 2010 edition, 10 lansoprazole enteric-coated tablets were extracted, and put into 100mL measuring bottles according to the quality standards of the first supplement of the Chinese Pharmacopoeia (2010 edition) Lansoprazole enteric-coated tablets , add 5mL of 0.1mol / L sodium hydroxide solution to ultrasonically disintegrate, add an appropriate amount of methanol-water (60:40) solution, ultrasonically dissolve, let cool, dilute to the mark with methanol-water (60:40) solution, Filtrate or centrifuge, take the subsequent filtrate or supernatant as the test solution, measure the content according to the high performance liquid chromatography under the content determination item, and the content uniformity limit is ±...
experiment example 2
[0073] This experimental example relates to the release test of lansoprazole enteric-coated tablets prepared in Example 1 of the present invention.
[0074] According to the Chinese Pharmacopoeia (2010 edition), the first supplement of this lansoprazole enteric-coated tablet quality standard, the release inspection method, get this product, according to the release assay (appendix X D second method), adopt the dissolution assay (appendix X C first method) device, using 1000mL of hydrochloric acid solution (9→1000) as the release medium, the rotation speed is 100 rpm, operate according to the law, after 120 minutes, immediately raise the rotating basket out of the liquid level, discard the hydrochloric acid solution, immediately Add 1000mL of phosphate buffer (pH6.8) preheated to 37°C, and continue to operate according to the law. After 45 minutes, take the solution and filter it, accurately measure 5mL of the filtrate, and accurately add 0.15mol / L sodium hydroxide solution 1mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com