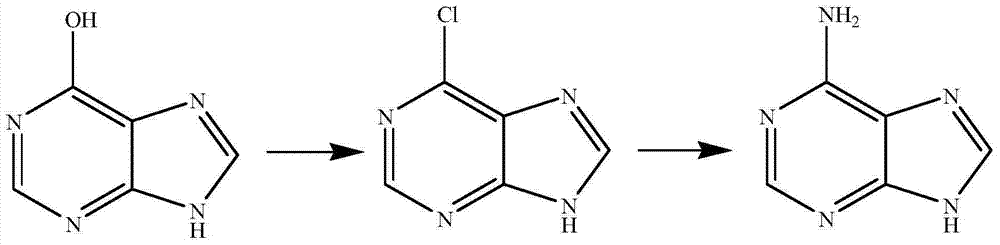
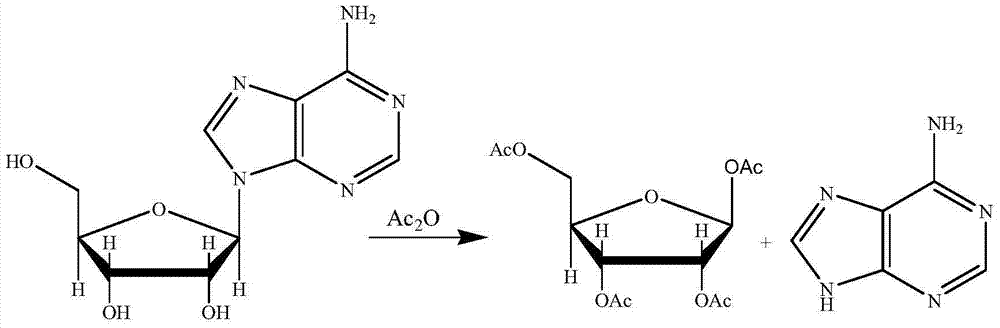
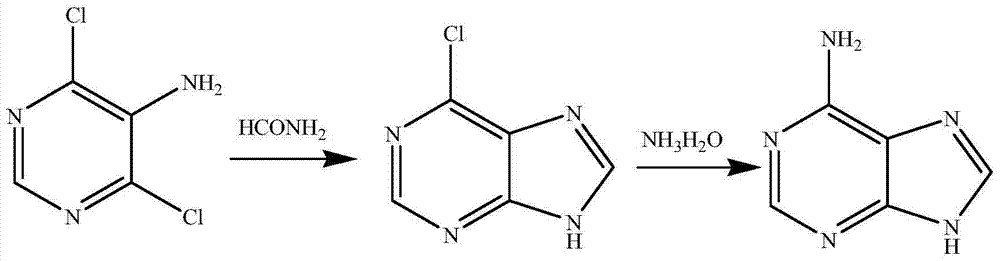
Synthetic method for adenine
A synthesis method and adenine technology are applied in the field of chemical synthesis routes of adenine, which can solve the problems of long synthesis routes, high raw material prices, unsuitability for large-scale production and the like, and achieve the effect of easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Step 1: Synthesis of 4,6-diamino-2-mercaptopyrimidine
[0086] Add 75 g of sodium ethoxide, 80 g of thiourea and 66 g of malononitrile into a 1000 mL three-necked flask, add 500 mL of absolute ethanol, stir, and heat to boiling. Reflux for 2h. Stop heating. Filter after cooling to obtain 4,6-diamino-2-mercaptopyrimidine filter cake, which is directly put into the next reaction. The yield is 75% based on malononitrile.
[0087] Step 2: Synthesis of 4,6-diamino-2-sulfinylpyrimidine
[0088] Dissolve the 4,6-diamino-2-mercaptopyrimidine filter cake obtained in step 1 in 1.0L 1mol / L sodium hydroxide solution, cool to -10°C, and add 150mL 30%H dropwise 2 O 2 The solution was stirred for 20 minutes, and then the pH of the reaction solution was adjusted to 4.5-5.0 with acetic acid. Filter to obtain 4,6-diamino-2-sulfinyl pyrimidine filter cake, which is directly used in the next reaction. The yield is calculated as 100%.
[0089] Step 3: Synthesis of 4,6-diaminopyrimidine
[0090]...
Embodiment 2
[0098] Step 1: The amount of ethanol was changed to 0.8L, and the other conditions were the same as step 1 in Example 1.
[0099] The synthesis reaction temperature of 4,6-diamino-2-sulfinopyrimidine was changed to -5°C, and other conditions were the same as step 2 of Example 1.
[0100] Step 3: The synthesis of 4,6-diaminopyrimidine is the same as step 3 in Example 1.
[0101] The synthesis of 4,6-diamino-5-nitrosopyrimidine is the same as step 4 in Example 1.
[0102] Step 5: Synthesis of 4,5,6-triaminopyrimidine
[0103] The moist 4,6-diamino-5-nitrosopyrimidine obtained in step 4 was added to an autoclave containing 400 mL of ethanol, 8 g of Raney nickel was added, hydrogen gas was added, stirred at room temperature for 10.0 h, filtered, and the filtrate was evaporated Remove methanol. The obtained 4,5,6-triaminopyrimidine was directly used in the next reaction. The yield is based on 95%.
[0104] Step 6: The cyclization agent used was changed to 0.8L formic acid, and other condit...
Embodiment 3
[0106] The synthesis of 4,6-diamino-2-mercaptopyrimidine was the same as step 1 in Example 1.
[0107] Step 2: The concentration of sodium hydroxide is changed to 1.2 mol / L, and other conditions are the same as step 2 of Example 1.
[0108] The synthesis of 4,6-diaminopyrimidine is the same as step 3 in Example 1.
[0109] Step 4: The input amount of sodium nitrite is changed to 164g, and the other conditions are the same as step 4 of embodiment 1.
[0110] Step 5: Synthesis of 4,5,6-triaminopyrimidine.
[0111] The moist 4,6-diamino-5-nitrosopyrimidine obtained in step 4 was added to an autoclave containing 400 mL of methanol, 4 g of 5% palladium carbon was added, hydrogen gas was added, and the mixture was stirred at room temperature for 8.0 h. After the reaction was completed, it was filtered, and the filtrate was evaporated to remove methanol. The obtained 4,5,6-triaminopyrimidine was directly used in the next reaction. The yield is based on 95%.
[0112] Step 6: The cyclization a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com