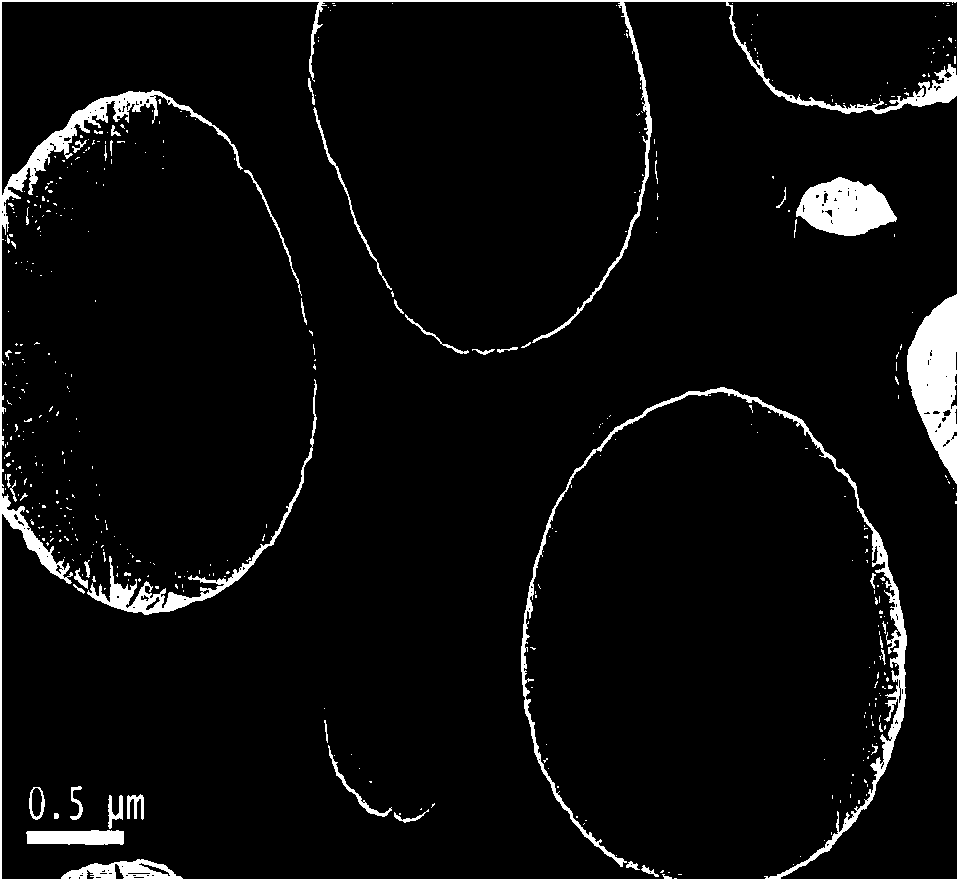
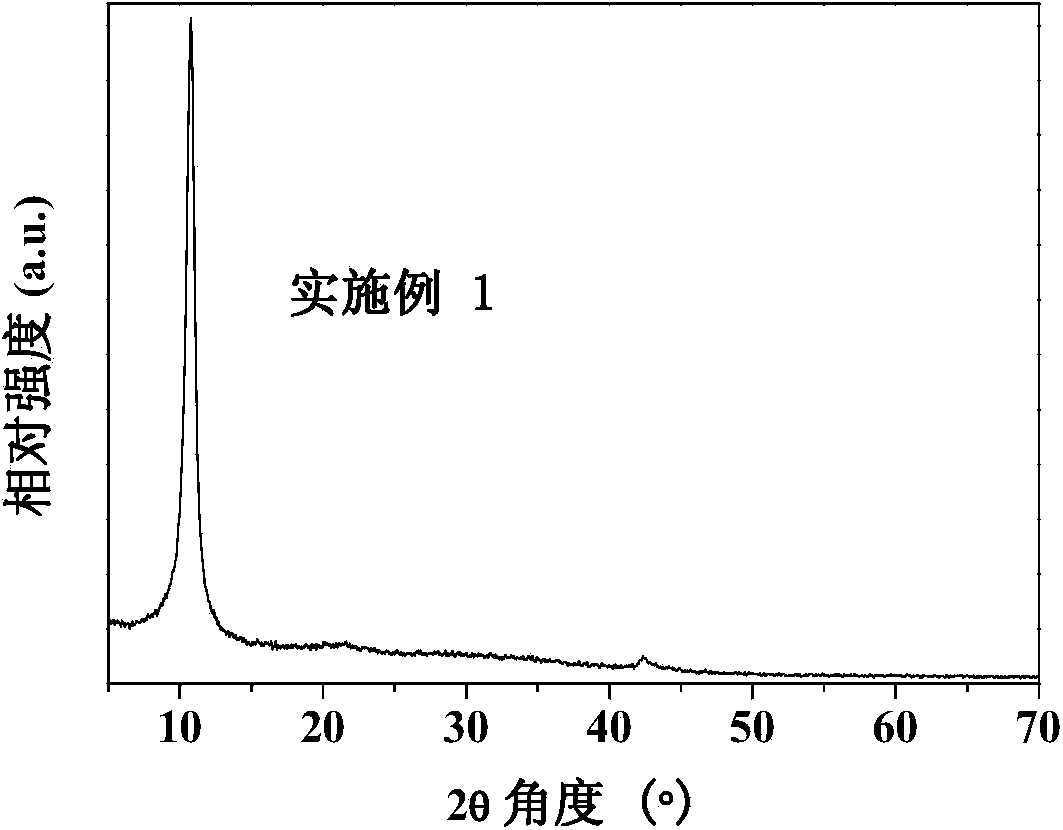
Nitrogen-doped graphene oxide material and preparation method thereof
A nitrogen doping, graphene technology, applied in chemical instruments and methods, inorganic chemistry, carbon compounds, etc., can solve problems such as unknown impact, achieve the effect of expanding application fields and avoiding irreversible stacking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 0.6 g of expanded graphite powder in a crucible, move it to a muffle furnace, and keep it at 400° C. for 0.5 h in an air atmosphere to obtain pre-expanded graphite powder. The obtained graphite powder was dispersed in isopropanol solution, ultrasonicated for 1 h, then 208 μL of pyrrole (Py) was added, stirred vigorously for 0.5 h, 1.3692 g of ammonium persulfate was added for in-situ polymerization for 4 h, and the product was filtered and washed with ethanol and deionized water in sequence After that, it was dried in a vacuum oven at 60°C for 24 hours. The obtained dried samples were calcined at 500° C. for 2 h in an argon (Ar) atmosphere to obtain nitrogen-doped graphite powder. Gained nitrogen-doped graphite powder was added to 80°C containing 30mL of concentrated sulfuric acid, 0.3g potassium persulfate (K 2 S 2 o 8 ) and 0.3g phosphorus pentoxide (P 2 o 5 ) in a round-bottomed flask for 6 h, then cooled to room temperature, the resulting blue solid was wa...
Embodiment 2
[0026]Take 0.6 g of expanded graphite powder in a crucible, move it to a muffle furnace, and keep it at 300° C. for 2 hours in an air atmosphere to obtain pre-expanded graphite powder. The obtained graphite powder was further placed in an argon-ammonia gas mixture with a concentration of 1% and calcined at 700° C. for 48 hours to obtain nitrogen-doped graphite powder. Gained nitrogen-doped graphite powder was added to a condensing reflux device containing 50mL concentrated nitric acid and 32g potassium chlorate (KClO 3 ) solution in a round bottom flask, the temperature was raised to 60° C. for 12 h, and then the product was washed with deionized water. The resulting product was finally dialyzed with a dialysis bag with a molecular weight cut-off of 3500 for 7 days to remove residual ions, and the resulting solution was ultrasonically dispersed to obtain nitrogen-doped graphene oxide.
Embodiment 3
[0028] Take 0.6 g of expanded graphite powder in a crucible, move it to a muffle furnace, and keep it at 500° C. for 3 hours in an air atmosphere to obtain pre-expanded graphite powder. The obtained graphite powder was further placed in 30% helium-ammonia mixed gas and calcined at 1200° C. for 24 hours to obtain nitrogen-doped graphite powder. Gained nitrogen-doped graphite powder was added to 80°C containing 30 mL of concentrated sulfuric acid, 0.3 g of potassium persulfate (K 2 S 2 o 8 ) and 0.3g phosphorus pentoxide (P 2 o 5 ) in a round-bottomed flask for 6 h, then cooled to room temperature, the resulting blue solid was washed and filtered with ultrapure water until the pH was 7, and then dried in a vacuum oven at 50°C, and then the resulting dry solid was slowly added to the In the 50mL concentrated sulfuric acid solution in the water bath, slowly add 4.2g potassium permanganate (KMnO 4 ) and stir until the temperature no longer rises, then keep the reaction tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com