Recombinant high-temperature pullulanase and preparation method thereof
A technology of pullulanase and high temperature, which is applied in the field of bioengineering, can solve the problem that proteins cannot be folded into mature proteins with correct conformation, etc., and achieve the effects of reducing saccharification time, high biological activity and improving saccharification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Construction of high-temperature pullulanase engineering bacteria and expression of its enzyme
[0065] 1. Primer design and PCR method to obtain high-temperature pullulanase target gene and preparation of recombinant vector
[0066] The genome of Thermus scotoductus SA-01 strain has a predicted sequence that may encode a pullulanase gene. The selected nucleotide sequence is shown in the sequence table SEQ ID NO: 1, and the gene is named pullu. The gene of the enzyme is amplified from the genome DNA of Thermus scotoductus SA-01 bacteria by PCR method. Primers were designed according to the gene sequence and restriction enzyme sites in the vector, and were commissioned to be synthesized by Beijing Aoke Dingsheng Biotechnology Co., Ltd. Upstream primer: 5'ATG CCATGG CCTGGTACGAGGGCGCTTTCTT’, the underlined part is the restriction site of NcoI; downstream primer: 5’CCG CTCGAGG ACCTCCACCCAGATGGCCACG3', the underlined part is the restriction site of Xho I; the...
Embodiment 2
[0077] Embodiment 2: Activity analysis of recombinant high-temperature pullulanase
[0078] Recombinant high-temperature pullulanase activity was analyzed by DNS method: at pH 6.5, 70°C, 500 μL reaction system included: 250 μL 1% pullulan substrate (g / 100mL) and 240 μL buffer (50mM) preheated After 10 minutes, add 10 μL of appropriately diluted enzyme solution to react for 5 minutes, add 750 μL of DNS to terminate the reaction, cook in boiling water for 5 minutes, measure the OD value at 540 nm after cooling, and use glucose as the standard curve to calculate the enzyme activity. One enzyme activity unit (U) is defined as the amount of enzyme required to degrade 1% pullulan solution per minute and release 1 μmol of reducing sugar under given conditions.
Embodiment 3
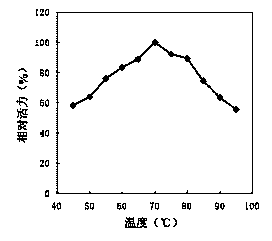
[0079] Example 3: Determination of the properties of recombinant high-temperature pullulanase
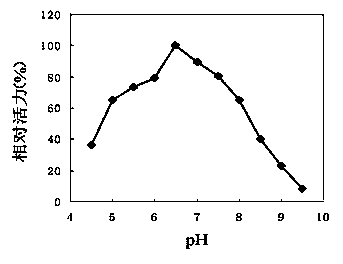
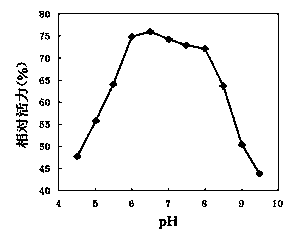
[0080] 1. The method for determining the optimum pH and pH stability of the recombinant high-temperature pullulanase is as follows: the recombinant high-temperature pullulanase purified in Example 1 is subjected to enzymatic reactions at different pHs to determine its optimum pH. Using 0.5% pullulan as a substrate, using the reaction system in Example 2, 50 mM potassium hydrogen phthalate-imidazole buffer solution with different pH was used to measure pullulanase activity at 65°C. The result is as figure 2 Shown, show that the optimum pH of pullulanase is 6.5. High-temperature pullulanase was treated at 70°C for 60min in the above-mentioned different pH buffers, and then the residual enzyme activity was measured at 70°C to study the pH stability of the enzyme. The result is as image 3 As shown, it shows that the high-temperature pullulanase is stable in the range of pH 6-8, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com