Method for extracting N6-(2-ethoxyl) adenosine from cordyceps militaris and application of N6-(2-ethoxyl) adenosine
A technology of hydroxyethyl and Cordyceps militaris, applied in chemical instruments and methods, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of long taking time, no anti-insomnia active monomer, large dosage, etc. problem, to achieve the effect of increasing extraction rate, reducing autonomic activity, and prolonging sleep time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Extraction of N6-(2-hydroxyethyl)adenosine in Cordyceps militaris
[0041] 1. Raw materials and materials:
[0042] The fruiting bodies of Cordyceps militaris were produced in this laboratory.
[0043] 2. Reagents:
[0044]Ultrapure water was produced by Millipore Water Purifier; 95% ethanol was purchased from Tianjin Beifang Tianyi Chemical Reagent Factory; chromatography-grade methanol was purchased from Honeywell Company.
[0045] 3. Instruments and equipment:
[0046] The ultrafine pulverizer was purchased from Shandong Sanqing Stainless Steel Equipment Co., Ltd.; the desktop refrigerated centrifuge was purchased from Thermo Company; the rotary evaporator was purchased from Shanghai Yarong Biochemical Instrument Factory; C18 chromatographic column (150×250mm) Jiangsu Hanbang Technology Co., Ltd.; Multifunctional Membrane Separation System Hangzhou Kaijie Membrane Separation Technology Co., Ltd.
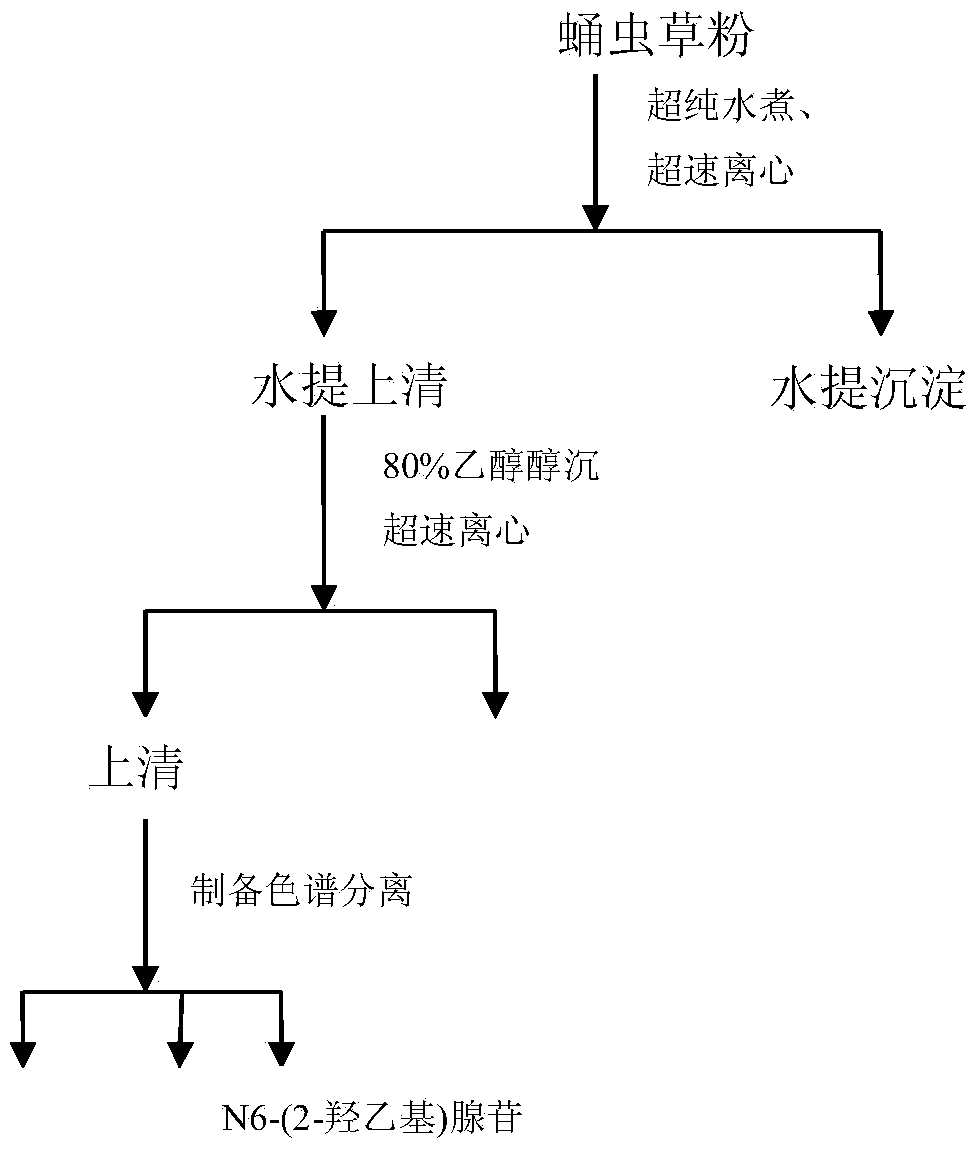
[0047] The extraction steps of N6-(2-hydroxyethyl)adeno...
Embodiment 2
[0056] Example 2: Confirmation of N6-(2-hydroxyethyl)adenosine
[0057] 1. Purity analysis by HPLC:
[0058] The target component N6-(2-hydroxyethyl)adenosine obtained in Example 1 was analyzed for purity by HPLC. The purity reached 98% through high performance liquid phase detection, and the HPLC spectrogram is shown in image 3 .
[0059] 2. Mass spectrometry analysis:
[0060] Positive ion mode electrospray mass spectrometry (ESI-MS) analysis of the target component N6-(2-hydroxyethyl)adenosine: Varian450GC-320TQ-M, Hanmeng Biotechnology (Tianjin) Co., Ltd., see the mass spectrum Figure 4 . Electrospray mass spectrometry [(+)-ESI-MS] gives a quasi-molecular ion peak m / z of 311.97 [M+H] + ; Prompt molecular composition is C 12 h 17 N 5 o 5 .
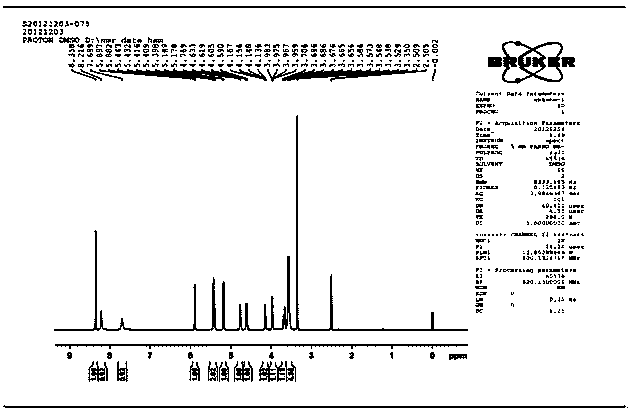
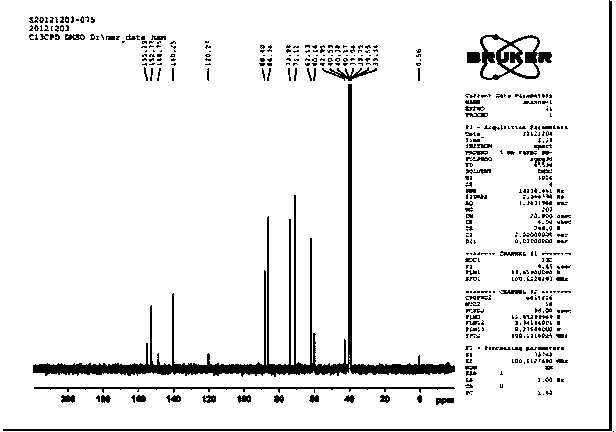
[0061] 3. NMR analysis:
[0062] NMR analysis of the target component N6-(2-hydroxyethyl)adenosine:
[0063] 1D 1 The data results in the HNMR spectrum (DMSO-d6, 400MHz) are as follows: δ: 8.358 (1H, s, H-8), 8.216 (1H, s...
Embodiment 3
[0067] Embodiment 3: N6-(2-hydroxyethyl) adenosine in anti-insomnia effect test
[0068] 1. Drugs and reagents:
[0069] Zaoren Anshen Capsules were purchased from Guizhou Tongjitang Pharmaceutical Co., Ltd.; Estazolam Tablets were purchased from Tianjin Pacific Pharmaceutical Co., Ltd.; pentobarbital sodium was packaged by Sigma.
[0070] 2. Test animals:
[0071] KM mice (18-22 g) (animal license number: SCXK (Beijing) 2012-0001, certificate number: 11400700020779) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0072] 3. Equipment and instruments
[0073] The syringe was purchased from Shanghai Zhiyu Medical Instrument Co., Ltd.; the stopwatch and EL electronic balance were purchased from Changzhou Tianzhiping Instrument Equipment Co., Ltd.
[0074] Get the N6-(2-hydroxyethyl)adenosine that embodiment 1 extracts, carry out anti-insomnia effect test, method step is as follows:
[0075] 1. Drug preparation:
[0076] N6-(2-hydroxyethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com