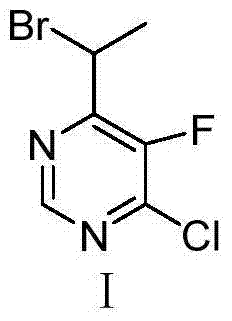
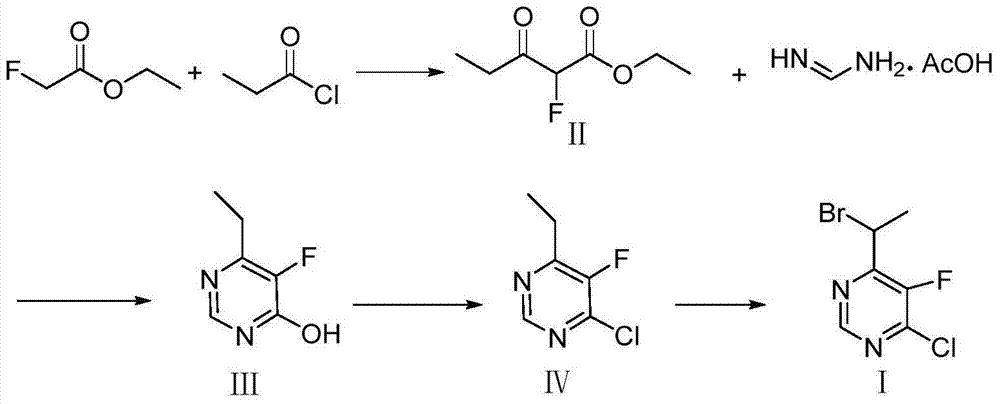
Method for synthesizing 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine
A technology of bromoethyl and synthetic methods, which is applied in the field of chemical drug synthesis, can solve problems such as high requirements for reaction equipment control and container pressure, large irritating odor at the production site, and high price of 5-fluorouracil. The effect of low requirements, elimination of potential safety hazards, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Step (1): Synthesis of ethyl 2-fluoropropionoacetate
[0035] In a 500mL three-necked flask, add 300mL THF, 30.8g sodium ethoxide, and 1.6g triethylamine, cool down to below 10°C, slowly add 31.8g ethyl 2-fluoroacetate, 55.2g propionyl chloride dropwise. Under reaction 16h. After the reaction was completed, THF was recovered by distillation under reduced pressure, and then 30 mL of water was added, extracted with 30 mL of ethyl acetate to obtain an organic phase, which was dried with anhydrous magnesium sulfate, and then rectified to obtain the product ethyl 2-fluoropropionoacetate 45.9 g, colorless liquid, yield 85%, gas chromatography purity 90%.
[0036] Step (2): Synthesis of 6-ethyl-5-fluoro-4-hydroxypyrimidine
[0037] In a 500mL three-necked flask, add 200mL of methanol, slowly add 27.5g of sodium methoxide, cool down to 10°C, add dropwise 45.9g of ethyl 2-fluoropropionoacetate prepared by the method in the above step (1), and add acetic acid in one go Formami...
Embodiment 2
[0043] Step (1): Synthesis of ethyl 2-fluoropropionoacetate
[0044] In a 500mL three-necked flask, add 300mL of methyl tert-butyl ether, 30.4g of sodium ethoxide, and 2.2g of triethylamine, cool down to below 10°C, slowly add 31.6g of ethyl 2-fluoroacetate, 54.9g of propionyl chloride dropwise, After that, react at 20°C for 14h. After the reaction was completed, methyl tert-butyl ether was recovered by distillation under reduced pressure, and then 30 mL of water was added, extracted with 30 mL of ethyl acetate to obtain an organic phase, which was dried with anhydrous magnesium sulfate, and then rectified to obtain the product 2-fluoropropane Ethyl acetoacetate 42.8g, colorless liquid, yield 78%, gas chromatography purity 88%.
[0045] Step (2): Synthesis of 6-ethyl-5-fluoro-4-hydroxypyrimidine
[0046] In a 500mL three-neck flask, add 200mL of ethanol, slowly add 25.1g of sodium methoxide, cool down to 10°C, add dropwise 42.8g of ethyl 2-fluoropropionoacetate prepared by t...
Embodiment 3
[0052] Step (1): Synthesis of ethyl 2-fluoropropionoacetate
[0053] In a 500mL three-necked flask, add 300mL of isopropyl ether, 17.8g of sodium hydride, and 2.8g of triethylamine, lower the temperature to below 10°C, slowly add 31.3g of ethyl 2-fluoroacetate, and 54.3g of propionyl chloride dropwise. React at 25°C for 12h. After the reaction, distill under reduced pressure to recover isopropyl ether, then add 30 mL of water, extract with 30 mL of ethyl acetate to obtain an organic phase, dry the organic phase with anhydrous magnesium sulfate, and then rectify to obtain the product ethyl 2-fluoropropionoacetate Ester 40.8g, colorless liquid, yield 77%, gas chromatography purity 89%.
[0054] Step (2): Synthesis of 6-ethyl-5-fluoro-4-hydroxypyrimidine
[0055] In a 500mL three-neck flask, add 200mL of ethanol, slowly add 30.5g of sodium ethoxide, cool down to 10°C, dropwise add 40.8g of ethyl 2-fluoropropionoacetate prepared by the method of the above step (1), and add Form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com