Preparation method of 4-methyl umbrella-shaped keto-beta-D-pyran galactoside
A technology of methylumbelliferyl and galactopyranoside, which is applied in the field of galactoside synthesis, can solve the problems of easy decomposition of bromide, unknown yield, and unfriendly waste liquid, and achieve simple post-processing, The effect of reducing production cost and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
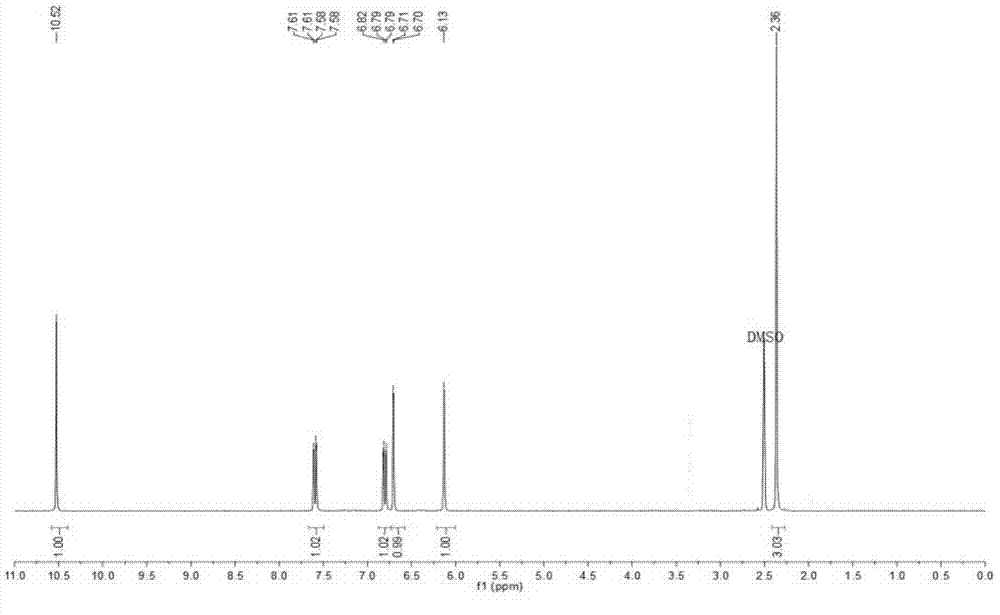
[0047] Weigh 5.5g of resorcinol and add it to 6.5g of ethyl acetoacetate, heat to dissolve and slowly add it dropwise to 50ml of concentrated sulfuric acid with a volume fraction of 75% (below 4°C). After the dropwise addition was completed and reacted at room temperature for 12 h, the reaction solution was poured into ice water and vigorously stirred, and a solid was precipitated, which was filtered by suction and washed with a large amount of ice water to obtain a pale yellow solid. The white crystalline powder of 4-methylumbelliferone was obtained by recrystallization from absolute ethanol, and the yield was 74.4%. That 1 H NMR spectrum seefigure 1 . 1 H NMR(300MHz,DMSO)δ10.52(s,1H,H-OH),7.59(dd,J=8.7,1.9Hz,1H,H-5),6.80(dd,J=8.7,2.3Hz,1H , H-6), 6.71 (d, J=2.3Hz, 1H, H-8), 6.13 (s, 1H, H-3), 2.36 (s, 3H, CH3).
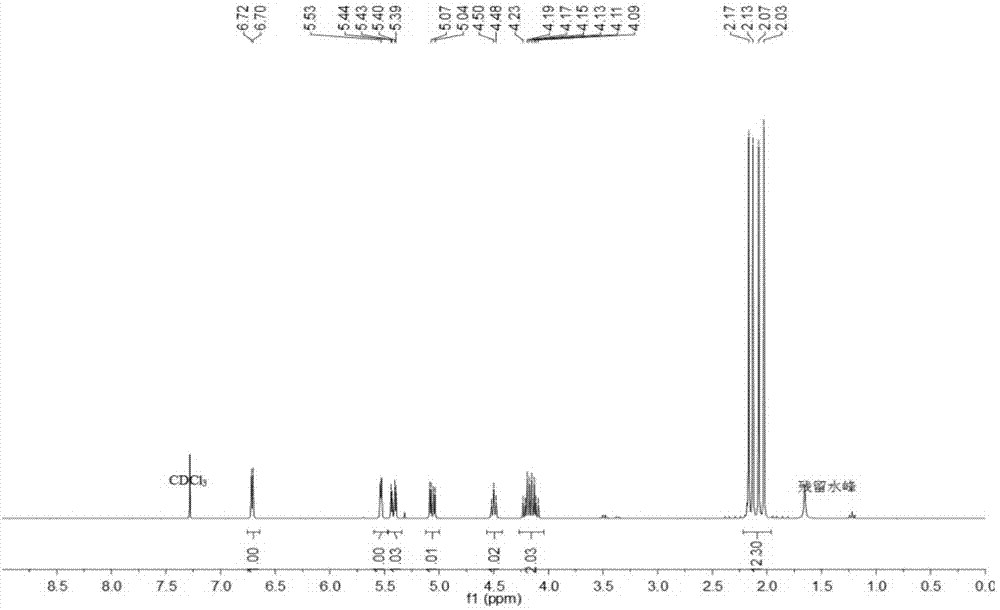
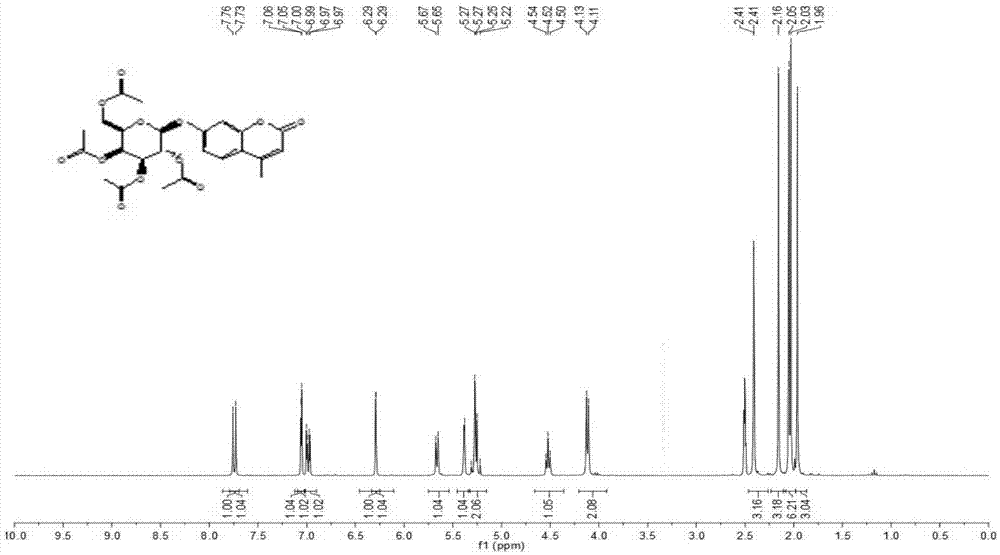
[0048] Weigh 5g of β-D-galactose pentaacetate, add 33%-35% hydrobromic acid-acetic acid solution 7.5-10ml, react at room temperature for 4h, pour the reaction so...
Embodiment 2
[0052] Example 2: Application of 4-methylumbelliferyl β-D-galactopyranoside in the detection of coliform bacteria
[0053] Experimental materials and sources
[0054] 1. The standard product 4-methylumbelliferone-β-D-galactopyranoside was purchased from APOLLO Technology Co., Ltd.
[0055] 2. Tryptone, Sodium Chloride, Potassium Monobasic Phosphate, Dipotassium Hydrogen Phosphate, and Sodium Lauryl Sulfate
[0056] 3. Escherichia coli ATCC25922, Citrobacter freundii ATCC8090, Enterobacter aerogenes CMCC45103, Klebsiella pheumoniae ATCC10031, Proteus vuigaris CMCC49027, Enterococcus 2 ATCC 2 faecalis Shigella flexneri CMCC51572 and Salmonella trphimurium ATCC14028 were purchased from Guangdong Huankai Microbiology Technology Co., Ltd., and the experimental samples were purchased from supermarkets.
[0057] 1. Pure bacteria detection
[0058] 1) Strains:
[0059] Inoculate the bacteria used in the experiment into the nutrient broth, culture at 37°C for 24 hours, and then it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com