Pyridyl cobalt phthalocyanine-cobalt compound/graphene composite material and preparation method thereof
A pyridyl cobalt phthalocyanine, composite material technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of poor activity and stability, noble metal nanocatalysts The production cost is high, and the synthesis method is simple and feasible, excellent catalytic activity, and good catalytic stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Disperse 0.5g of graphite and 0.5g of sodium nitrate in 42.32g of concentrated sulfuric acid, slowly add 3g of potassium permanganate under mechanical stirring at 0°C; stir in a water bath at 35°C for 1 hour; add 40g of water, and After stirring at 90°C for 30min, add 100g of water and 4.44g of 30% hydrogen peroxide (H 2 o 2 ) after suction filtration, washing with water and centrifugation until the centrifuged water is neutral, vacuum drying at 45° C. for 12 hours to obtain graphite oxide;
[0030] (2) Disperse 0.1 g of the graphite oxide in 100 g of water to form a graphite oxide aqueous solution; add 0.5 g of sodium polystyrene sulfonate after ultrasonic oscillation of the graphite oxide aqueous solution for 4 h and continue ultrasonication for 1 h, add 1.03 g of hydrazine hydrate React at 100°C for 24 hours, cool to room temperature, centrifuge, and wash with water and ethanol to obtain graphene.
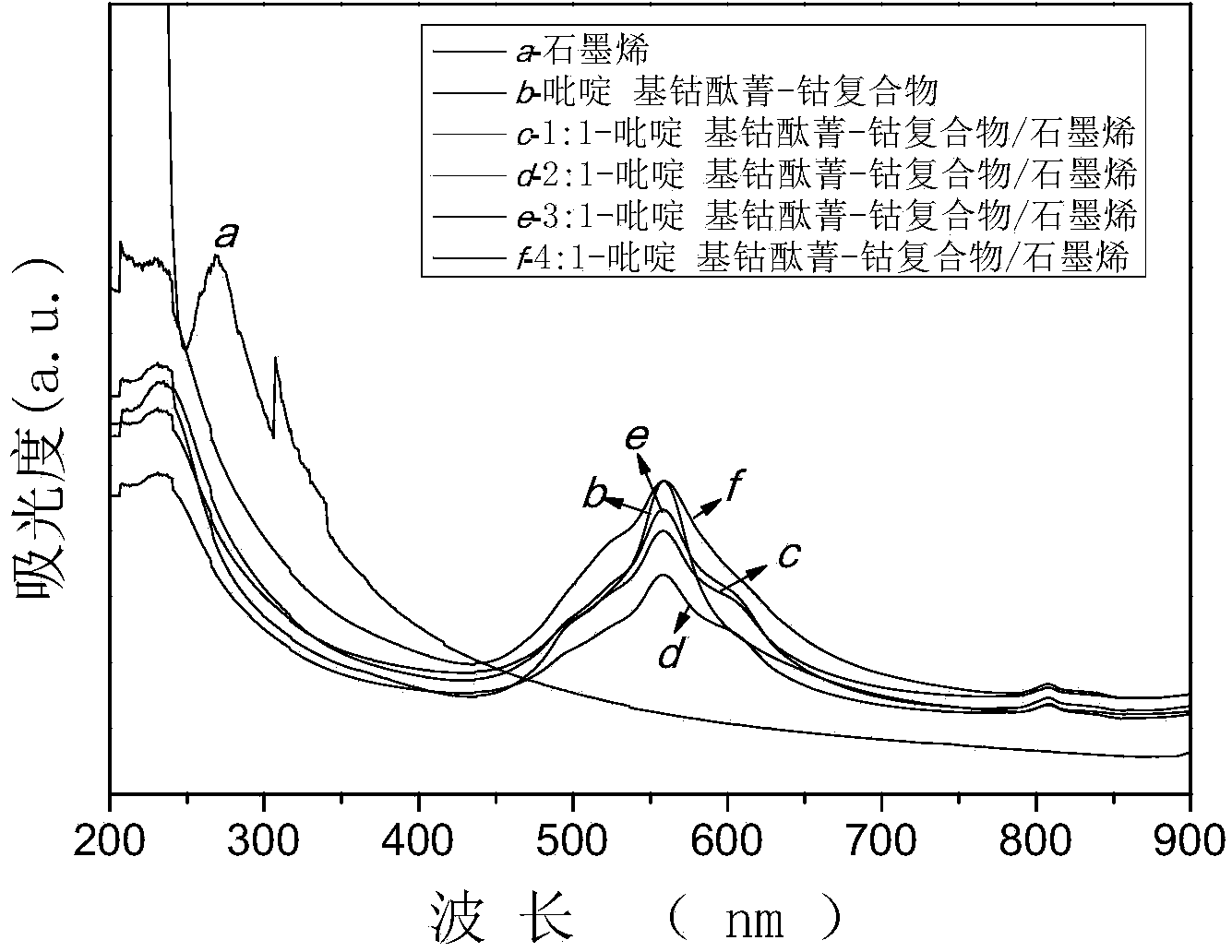
[0031] figure 1 Curve a is the ultraviolet-visible absorption...
Embodiment 2
[0033] Add 0.011mol of 4-hydroxypyridine and 0.01mol of 4-nitrophthalonitrile to 60mL of N,N-dimethylformamide (DMF), in N 2 Under the protection of air, add 0.044mol sodium carbonate in 3 batches, stir the reaction at 80°C for 8 hours, pour it into deionized water after cooling to room temperature, and filter to obtain powdery pyridyl dinitrile, which is washed with ethanol 3 times, vacuum-dried at 50°C for 12h.
Embodiment 3
[0035] Add 15 mg of pyridyl dinitrile monomer and 4.22 mg of cobalt acetate tetrahydrate to 12.22 g of n-pentanol, then add 0.102 g of DBU, ultrasonically disperse for 1 hour, transfer the mixture to a reaction kettle, and store at 160°C After reacting for 24 hours, naturally cool to room temperature after the reaction, wash the powder three times with water and ethanol respectively, and dry to obtain the pyridyl cobalt phthalocyanine-cobalt complex.
[0036] figure 1 Curve b is the ultraviolet-visible absorption spectrum of pyridyl cobalt phthalocyanine-cobalt complex, the characteristic absorption peak of pyridyl cobalt phthalocyanine: B band is at 329nm, Q band is at 598 and 658nm, and the absorption peak of Q band shows A phthalocyanine ring has been formed.
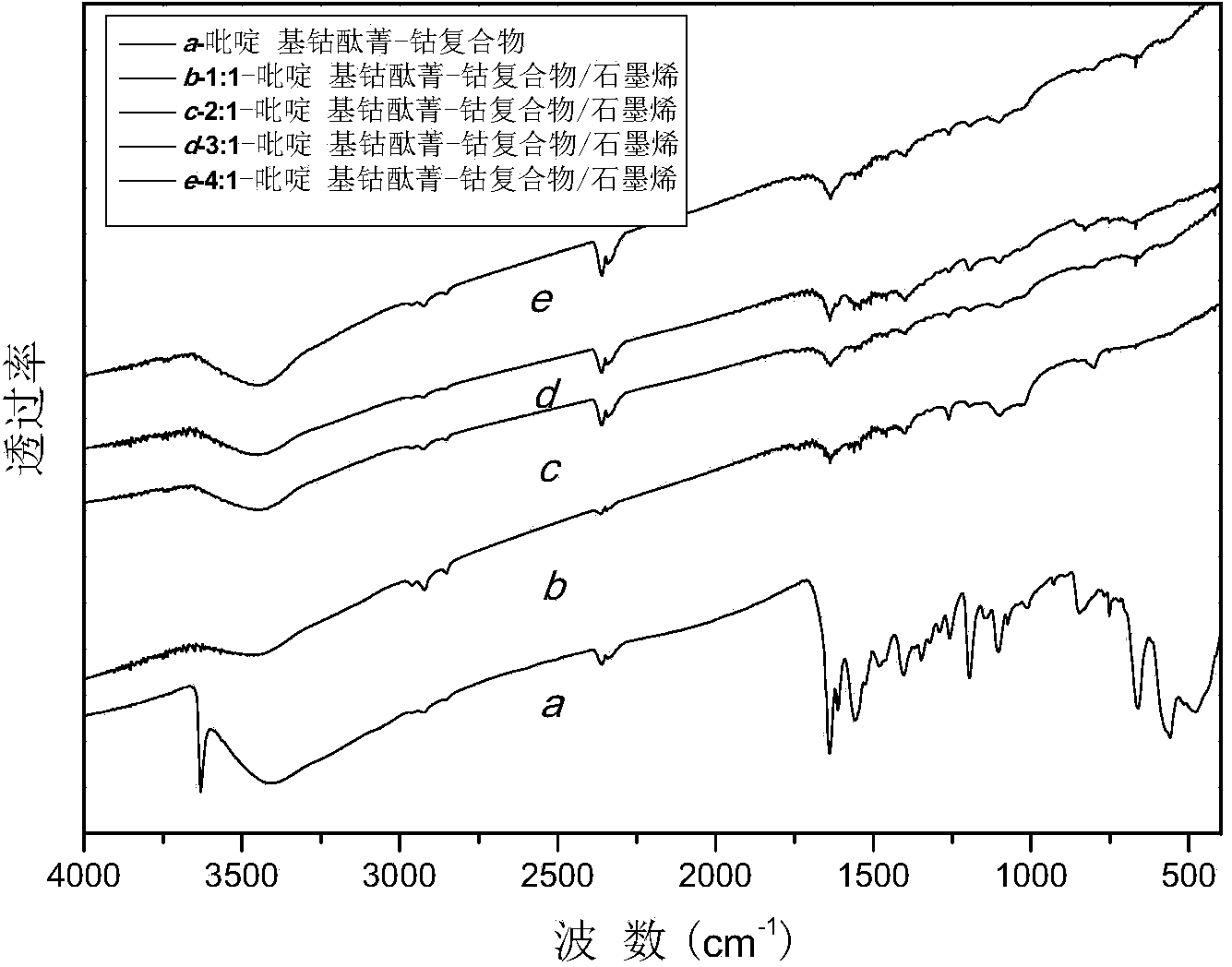
[0037] From the infrared spectrum of pyridyl cobalt phthalocyanine-cobalt complex ( figure 2 Spectral line a) can be seen, 750, 847 and 1100cm -1 The absorption peak at corresponds to the vibration absorption pea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peak current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com