Method for preparing 2-triazole-quinoline-4-carboxylic acid compound
A carboxylic acid compound and triazole technology are applied in the field of 2-triazole-quinoline-4-carboxylic acid compound and synthesis of 2-triazole-quinoline-4-carboxylic acid compound, which can solve the problem of yield low, long time, cumbersome steps and other problems, to achieve the effect of high yield, simple operation, good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
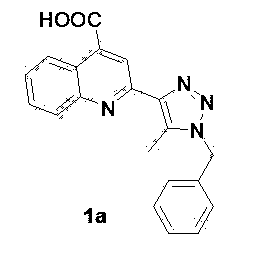
[0038] 2-[1-benzyl-5-methyl-1 H- 1,2,3-triazol-4-yl]-4-quinolinecarboxylic acids
[0039]
[0040] In a 25 mL small flask add 2.0 mmol benzyl bromide, 2.3 mmol NaN 3 Solid, 5 mL DMSO. The reaction solution was heated to 70°C under stirring, and after 10-12 hours of reaction, 1.5 mmol acetylacetone was added to the mixed solution, followed by the addition of 0.2 equivalents of diethylamine catalyst, 70 o Reflux and stir in an oil bath of C, track with a TLC plate during the reaction, add 1.0 mmol isatin to the flask after 24 hours, and add 1 mL of 8M potassium hydroxide at the same time, raise the reaction temperature to 80 ° C, stir and reflux 2~4 hours. After the reaction was complete, cool to room temperature, add 2 mL of water to the mixture, and adjust the pH to 6-7 with acetic acid solution. After precipitation occurs, filter the filter residue, wash with absolute ethanol for 2 to 3 times, and finally wash with anhydrous Na 2 SO 4 Dry, filter, remove the ...
Embodiment 2
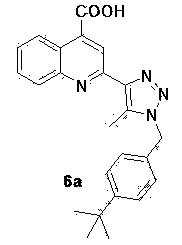
[0042] 2-[1-(4-Bromo-benzyl)-5-methyl-1 H- 1,2,3-triazol-4-yl]-4-quinolinecarboxylic acids
[0043]
[0044] In a 25 mL small flask add 2.0 mmol 4-bromo-benzyl bromide, 2.3 mmol NaN 3 Solid, 5 mL DMSO. The reaction solution was heated to 70°C under stirring, and after 10-12 hours of reaction, 1.5 mmol acetylacetone was added to the mixed solution, followed by the addition of 0.2 equivalents of diethylamine catalyst, 70 o Reflux and stir in an oil bath of C, track with a TLC plate during the reaction, add 1.0 mmol isatin to the flask after 24 hours, and add 1 mL of 8M potassium hydroxide at the same time, raise the reaction temperature to 80 ° C, stir and reflux 2~4 hours. After the reaction was complete, cool to room temperature, add 2 mL of water to the mixture, and adjust the pH to 6-7 with acetic acid solution. After precipitation occurs, filter the filter residue, wash with absolute ethanol for 2 to 3 times, and finally wash with anhydrous Na 2 SO 4 Dry, filt...
Embodiment 3
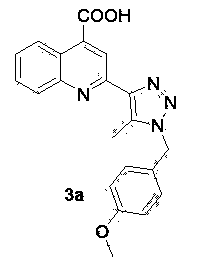
[0046] 2-[1-(4-Methoxy-benzyl)-5-methyl-1 H- 1,2,3-triazol-4-yl]-4-quinolinecarboxylic acids
[0047]
[0048] In a 25 mL small flask add 2.5 mmol 4-methoxy-benzyl bromide, 2.8 mmol NaN 3 Solid, 5 mL N,N-dimethylformamide. The reaction solution was heated to 70°C under stirring, and after 10-12 hours of reaction, 1.5 mmol acetylacetone was added to the mixed solution, followed by the addition of 0.2 equivalents of diethylamine catalyst, 70 o Reflux and stir in an oil bath of C, track with a TLC plate during the reaction, add 1.0 mmol isatin to the flask after 24 hours, and add 1 mL of 8M sodium hydroxide at the same time, raise the reaction temperature to 80 ° C, stir and reflux 2~4 hours. After the reaction was complete, cool to room temperature, add 2 mL of water to the mixture, and adjust the pH to 6-7 with acetic acid solution. After precipitation occurs, filter the filter residue, wash with absolute ethanol for 2 to 3 times, and finally wash with anhydrous N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com