Metal ions adsorbed by bentonite and regenerating method thereof
A technology of metal ions and bentonite, applied in the direction of separation methods, filter regeneration, chemical instruments and methods, etc., can solve the problems of a large amount of waste acid or waste alkali, danger, increasing the amount of bentonite and cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
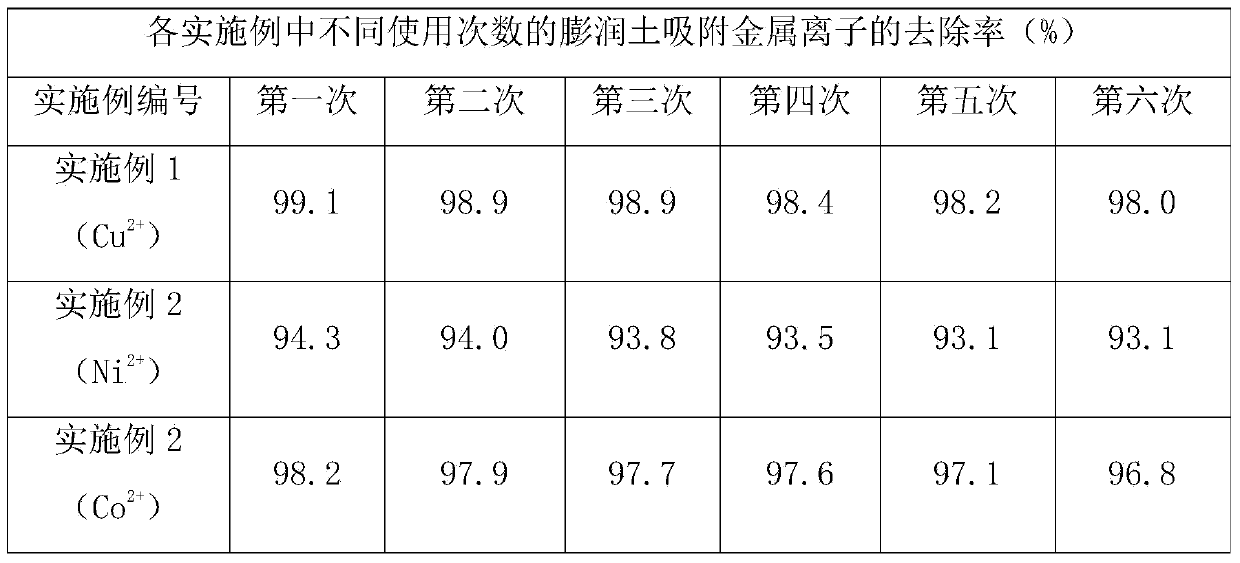
Embodiment 1
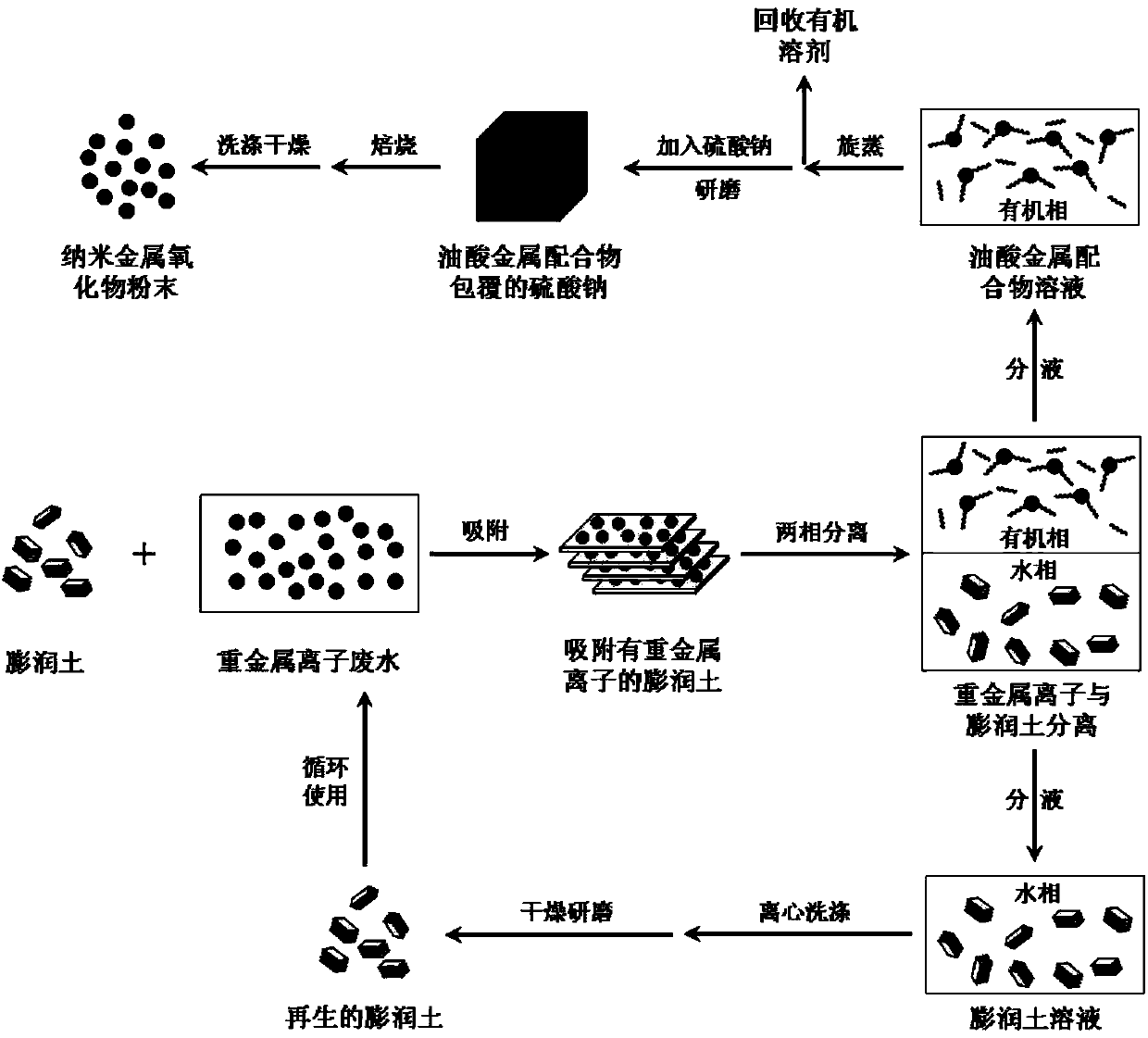
[0020] To 1000mL100mg L -1 15g of calcium-based bentonite was added to the copper sulfate solution, and after being adsorbed for 8 hours under stirring conditions, it was centrifuged, and the remaining Cu in the solution was determined by atomic adsorption spectrometry. 2+ concentration. Then, redisperse the obtained bentonite in 300mL of distilled water, then add 150mL of ethanol, 150mL of n-ethane and 1.5g of sodium oleate, reflux and stir at 70°C for 2h, let it cool down, then transfer the solution system to the liquid separation In the funnel, separate the water phase and the oil phase; centrifuge the lower water phase, wash the precipitate with water and centrifuge again, dry it at 60°C for 12 hours and grind it to obtain the regenerated bentonite; wash the upper oil phase with distilled water, Stand still, remove the lower aqueous phase, repeat 2 or 3 times, and then spin evaporate at 60°C and recover the organic solvent, and at the same time obtain a blue-green viscous...
Embodiment 2
[0022] To 1000mL100mg L -1 15g of calcium-based bentonite was added to the nickel nitrate solution, and after being adsorbed for 8 hours under stirring conditions, it was centrifuged, and the remaining Ni in the solution was determined by atomic adsorption spectrometry. 2+concentration, then redisperse the obtained bentonite in 300mL distilled water, then add 150mL ethanol, 150mL n-ethane and 1.5g sodium oleate, reflux and stir at 70°C for 2h, let it stand until cooling, then transfer the solution system to In the liquid funnel, separate the water phase and the oil phase; centrifuge the lower water phase, wash the precipitate with water and centrifuge again, dry it at 60°C for 12 hours and grind it to obtain the regenerated bentonite; wash the upper oil phase with distilled water , stand still, remove the lower aqueous phase, repeat for 2 or 3 times, then spin evaporate at 60°C and recover the organic solvent, and at the same time obtain green viscous nickel oleate; after that...
Embodiment 3
[0024] To 1000mL100mg L -1 15g of calcium-based bentonite was added to the cobaltous chloride solution, and after being adsorbed for 8 hours under stirring conditions, it was centrifuged, and the remaining Co in the solution was determined by atomic adsorption spectrometry. 2+ concentration, then redisperse the obtained bentonite in 300mL of distilled water, then add 150mL of ethanol, 150mL of n-ethane and 1.5g of sodium oleate, reflux and stir at 70°C for 2h, let it stand until it cools down, then transfer the solution system to In a separatory funnel, separate the water phase and the oil phase; centrifuge the lower water phase, wash the precipitate with water and centrifuge again, dry it at 60°C for 12 hours and grind it to obtain the regenerated bentonite; wash the upper oil phase with distilled water Washing, standing still, separating the lower aqueous phase, repeating 2 or 3 times, then rotating at 60 ° C and recovering the organic solvent, and simultaneously obtaining p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com