Bleomycin derivative separating and purifying method
A technology of bleomycin family and purification method, which is applied in the fields of peptide preparation methods, chemical instruments and methods, organic chemistry, etc., and can solve problems such as no activity, low toxicity, and low quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Both Streptomyces chrysogenum SB9001 and the genetically engineered mutant strain obtained in the experiment were cultured on ISP4 agar solid medium at 30°C to obtain spores. Streptomyces verticillium ATCC15003 was grown in TSBY liquid medium containing 0.5% glycine at 28°C and 250rpm to obtain genomic DNA. E. coli was grown in LB liquid medium at 37°C and 250rpm for routine plasmid cloning, BAC library establishment.
[0057] 1) Construction of Streptomyces aeruginosa SB9025 mutant strain
[0058]A 10kb DNA fragment containing the complete zbmVIII gene was isolated from the cosmid containing a part of the zobermycin biosynthetic gene cluster zbm, and the 10kb DNA fragment was cloned into the Litmus28 plasmid through the BamHI and BglII restriction sites . The obtained plasmid was digested with SbfI to remove the insert fragment of about 3.7 kb size, and then the plasmid was self-ligated to complete the knockout of the zbmVIII gene. The modified DNA insert was digest...
Embodiment 2
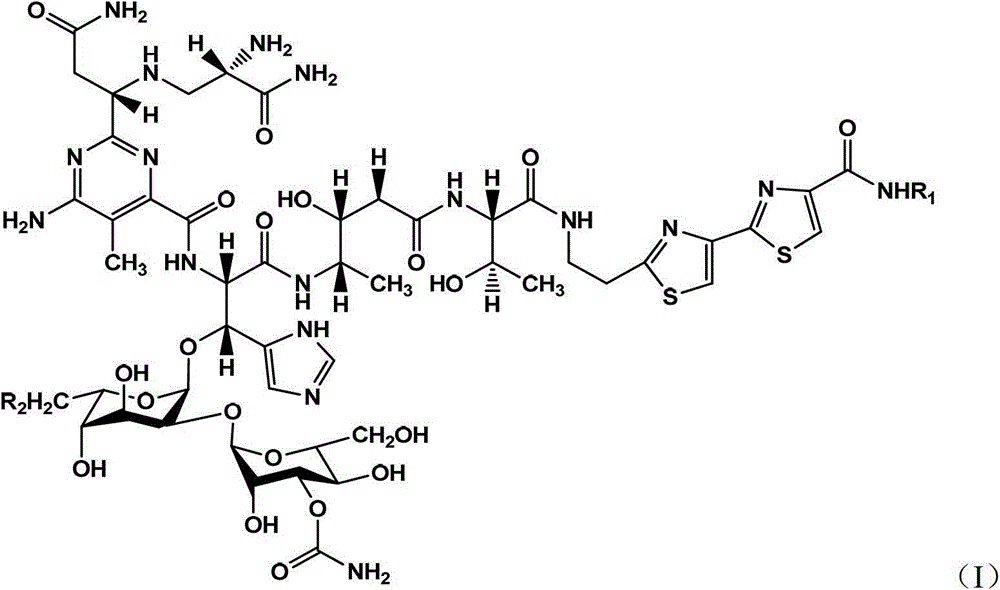
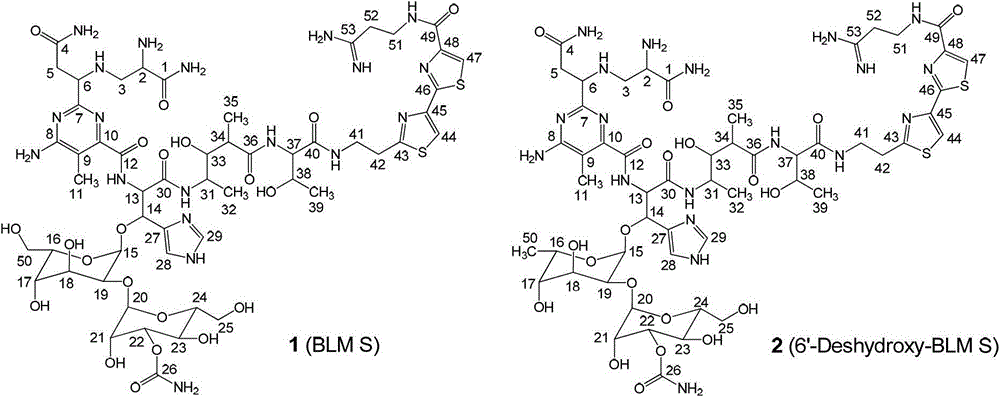
[0065] Separation, purification and detection of novel bleomycin analogs BLM S (Tinci 102, compound 1) and 6'-dehydroxy-BLM S (Tinci 103, compound 2)
[0066] The supernatant solution obtained after centrifugation of the fermentation broth was subjected to high performance liquid phase (HPLC) detection and analysis. Mobile phase A was 99.9% deionized water and 0.1% acetic acid, mobile phase B was 99.9% methanol and 0.1% acetic acid, and the flow rate was 0.8 mL. / min, and the wavelength of the UV detector is 300 nm. The linear gradient analysis program was: 0-30 minutes, 100%A / 0%B to 0%A / 100%B. The remaining supernatant was adjusted to pH 7.0 with 1N hydrochloric acid and loaded into Amberlite IRC50 (NH 4 + type) resin column, rinsed with 10 column volumes of deionized water, and eluted with 2 liters of 20% ammonium acetate solution. The resulting eluent was mixed with Diaion HP-20 resin and vortexed gently for 45 minutes at room temperature for adsorption, then the HP-20 r...
Embodiment 3
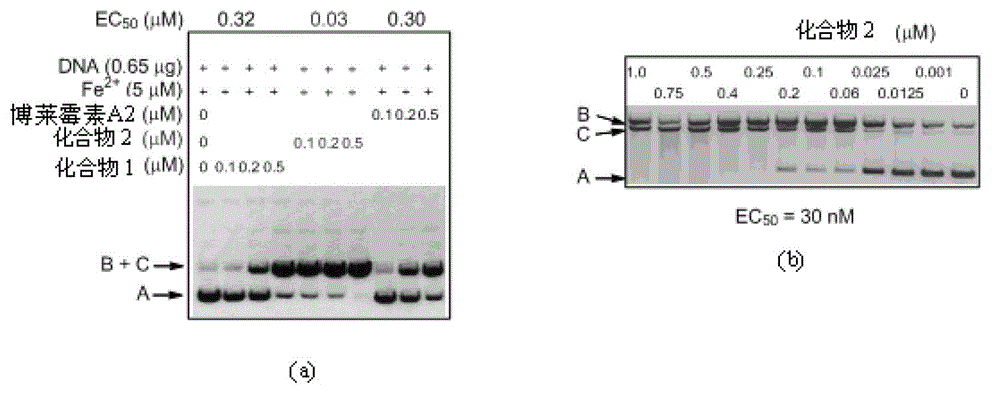
[0080] DNA cleavage activity test of BLM S (Tinci 102, compound 1) and 6'-dehydroxy-BLM S (Tinci 103, compound 2)
[0081] in Fe 2+ In the presence of BLM S, 6'-dehydroxy-BLM S and bleomycin A, respectively 2 (BLM A 2 ) were tested to compare the biological activity. Based on the analysis of the plasmid relaxation activity of the supercoiled plasmid DNA pBluescript II SK(+), single-strand cleavage mediated by bleomycin family compounds firstly transformed the supercoiled plasmid DNA (form A) into an open-circular plasmid DNA (form B). , followed by double-strand cleavage into linear plasmid DNA (form C). The total reaction volume of the DNA cleavage activity test experiment was 10 μl, which contained 25 mM Tris-HCl buffer (pH 7.5), about 0.65 μg of pBluescriptSK II(+) plasmid DNA, 5 μM Fe(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O (at 1 mM H 2 SO 4 freshly prepared in solution) and concentrations of the active compounds to be tested (BLM S, 6'-dehydroxy-BLM S, and BLM A 2 ). The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com