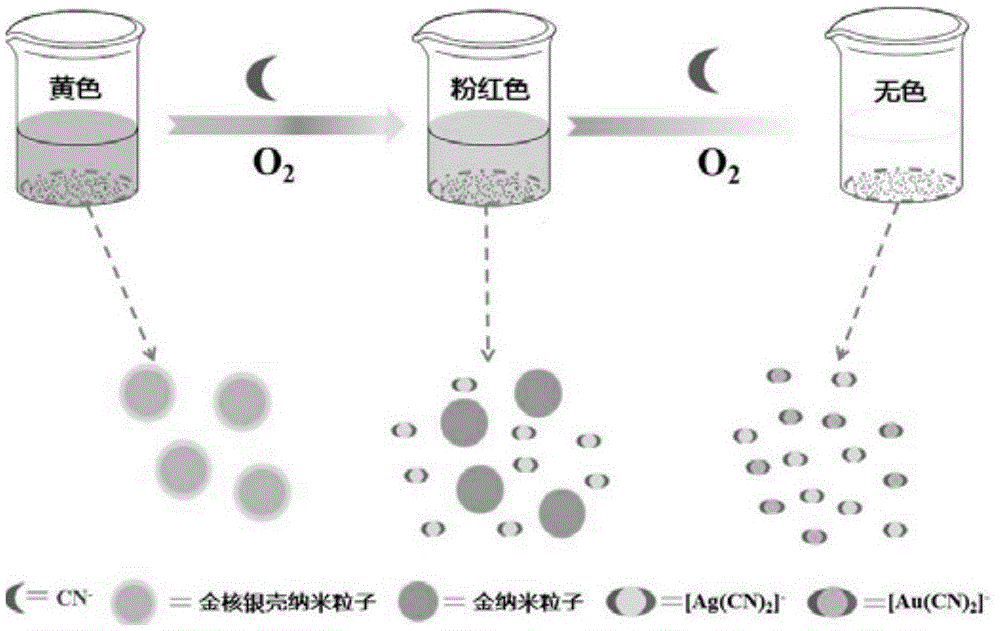
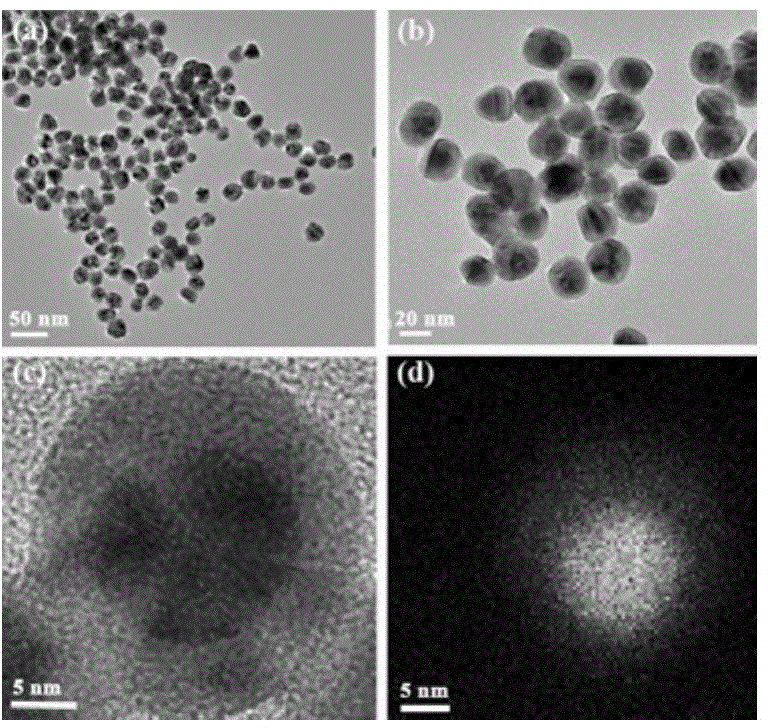
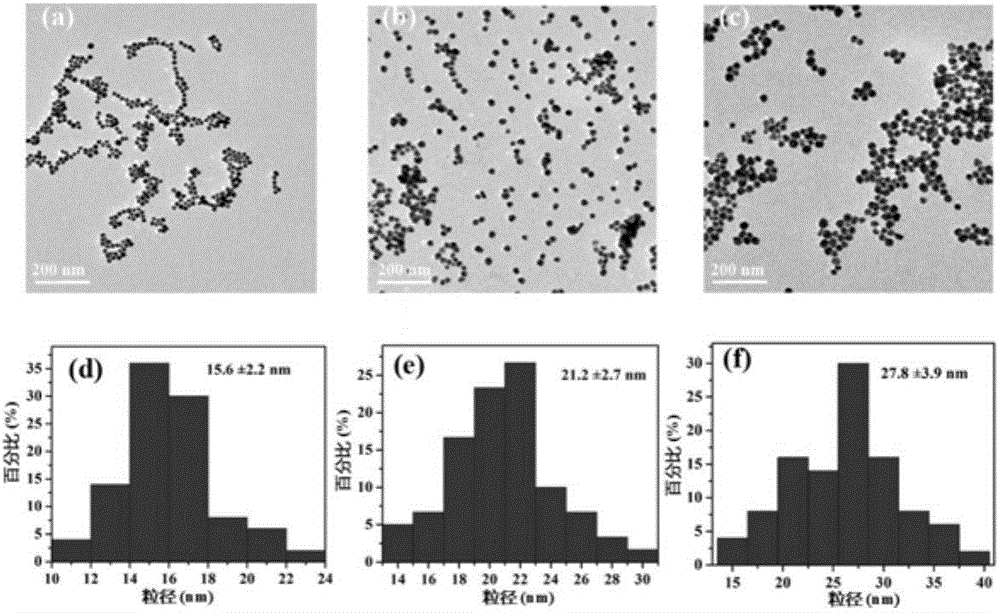
Gold-core silver-shell nanoprobe, preparation method thereof and application thereof in cyanide ion colorimetric detection
A nanoprobe, cyanide ion technology, applied in nanotechnology, analysis by chemical reaction of materials, material analysis by observing the impact on chemical indicators, etc., can solve the problem of low specificity, long reaction time, problems such as poor water compatibility, to achieve the effect of good selectivity, high sensitivity and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: The detection effect of the gold core silver shell nanoprobe prepared by the present invention on a series of cyanide ion solutions is given below. Prepare a series of cyanide ion solutions (0-160μM) with a concentration, add the gold core silver shell nanoparticle solution, and take pictures and scan the ultraviolet-visible spectrum after reacting at room temperature for 5 minutes. Figure 4 It shows that with the increase of cyanide concentration, the color of the solution changes from yellow to pink, and finally to colorless. According to the color change, the semi-quantitative detection of cyanide concentration can be realized. Figure 5 It shows that as the cyanide concentration increases, the absorbance at 394nm gradually decreases, and the change in absorbance has a good linear relationship with the cyanide concentration in the range of 0-100μM ( Image 6 ), the linear correlation coefficient reached 0.9984, and the minimum detection concentration was 0.4μ...
Embodiment 2
[0030] Example 2: The response time curves of the gold core silver shell nanoparticles of the present invention for detecting different concentrations of cyanide are given below. Prepare cyanide ion solutions of different concentrations, add gold core silver shell nanosols respectively, and monitor the relationship curve between the change in absorbance at 394 nm and the reaction time at room temperature using a spectrophotometer. by Figure 7 As shown, when the gold core silver shell nanosol is exposed to the cyanide ion environment, the absorbance value at 394nm decreases significantly within 1 min, and then tends to balance, indicating that the gold core silver shell nanoparticles of the present invention are used to detect cyanide ion It has the advantages of fast response speed and short response time.
Embodiment 3
[0031] Example 3: The following provides a comparison of the response effects of the gold core silver shell nanoparticles of the present invention on cyanide and 18 other types of anions. Figure 8 It is shown that the response signal of the gold core silver shell nanoparticles of the present invention to cyanide ions is 9.2-230 times that of all other 18 anions, indicating that the method has high specificity for cyanide ions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com