Thermophilic esterase and application thereof in degradation of PAEs (Phthalic Acid Esters)
A technology of esterase and biological enzyme, applied in the field of bioengineering, can solve the problems of little practical application value and poor stability, and achieve the effects of superior thermal stability, fast reaction speed and good stability of degradation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The plasmid construction of embodiment 1 thermophilic esterase gene
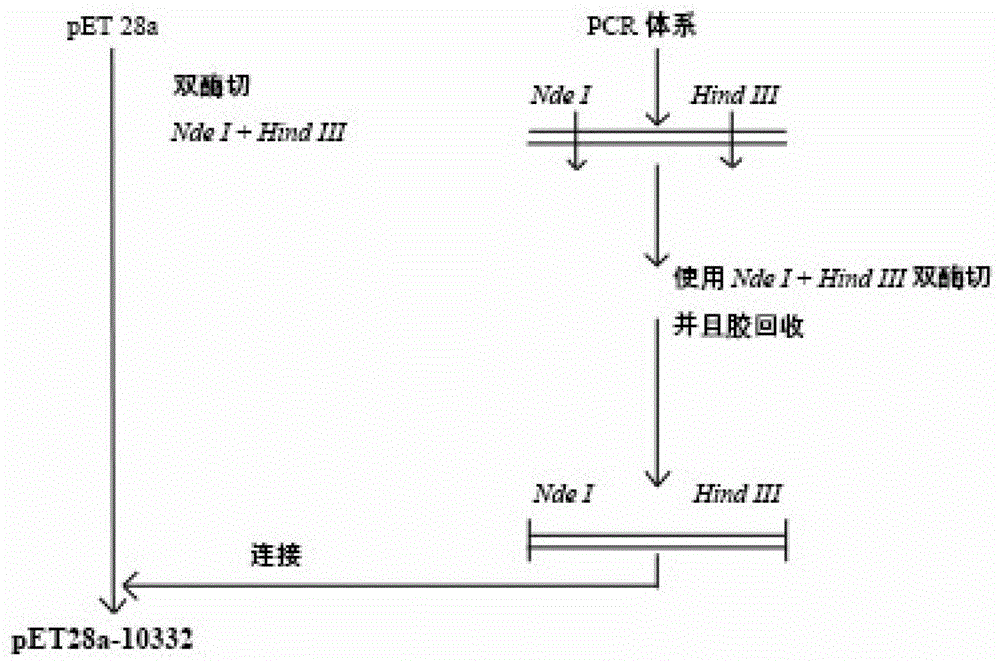
[0046] Using the genome of Sulfobacillus acidophilus as a template, and using 10332-Nde I-F and 10332-HindIII-R as forward and reverse primers, a 900bp gene fragment of 10332 encoding PAEs hydrolase was amplified by PCR. Glycogel electrophoresis check The obtained PCR product was connected to the pET28a vector and then transformed into Escherichia coli DH5α, and several white colonies were selected for identification. Screened by bacterial cell electrophoresis, the plasmid was extracted and verified by double enzyme digestion, and the correct pET28a-10332 recombinant plasmid containing the target gene was obtained, and the recombinant plasmid was transformed and stored in Escherichia coli BL21 (DE3), and the sequence was correct, successfully constructed The recombinant plasmid pET28a-10332 capable of heterologously expressing the target gene 10332 and its expression transformants. The schematic diag...
Embodiment 3
[0105] Example 3 Heterologous Expression and Isolation and Purification of Recombinant Escherichia coli
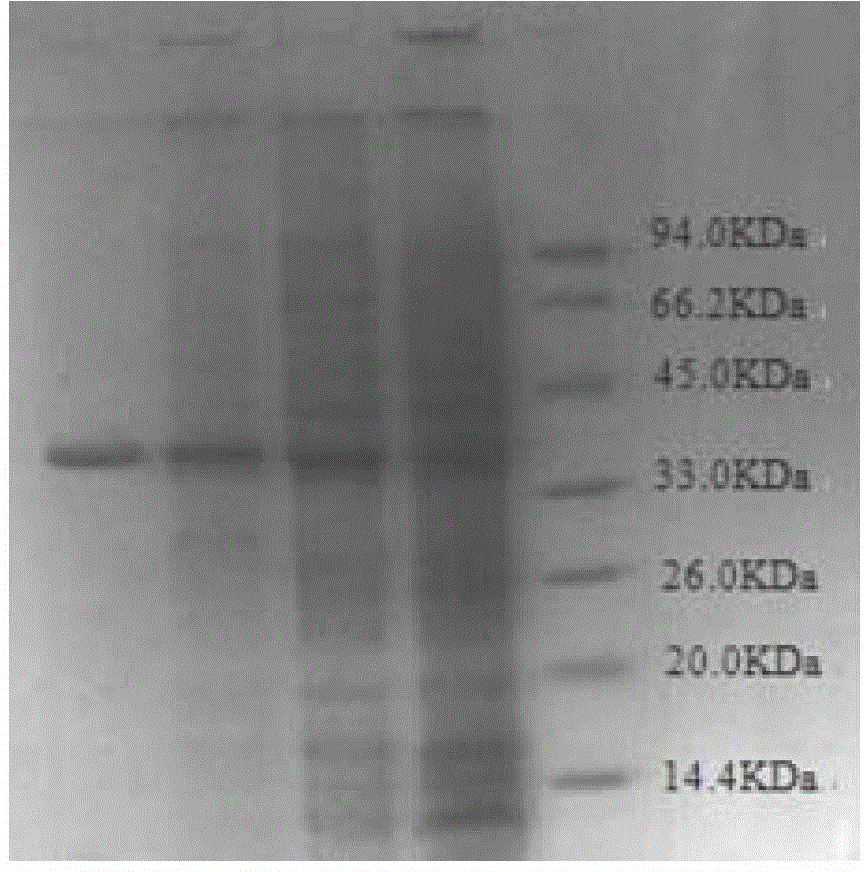
[0106] The expression transformant BL21(DE3) / pET28-10332 is cultivated, and the target protein can be soluble expressed by suitable temperature and suitable concentration of inducer IPTG, so that the enzyme can be produced in large quantities. Ultrasonic disruption is performed on the cells induced to express, and the supernatant after sonication is separated and purified by his-tag affinity chromatography separation method to obtain electrophoretic pure protein of thermophilic esterase with his-tag tag.
[0107] 1 Induced expression of foreign fragments in Escherichia coli
[0108] Place the expression transformant BL21(DE3) / pET28–10332 on a constant temperature plate at 37°C for 12 hours, pick a single colony in 5ml LB (containing 50μg / ml kanamycin) liquid medium, cultivate it for 8 hours, and make glycerol Keep a spare. Take 5 μl of the glycerol tube bacteria solution...
Embodiment 4
[0129] Example 4 Enzymatic properties and substrate spectrum of recombinant Escherichia coli expression product
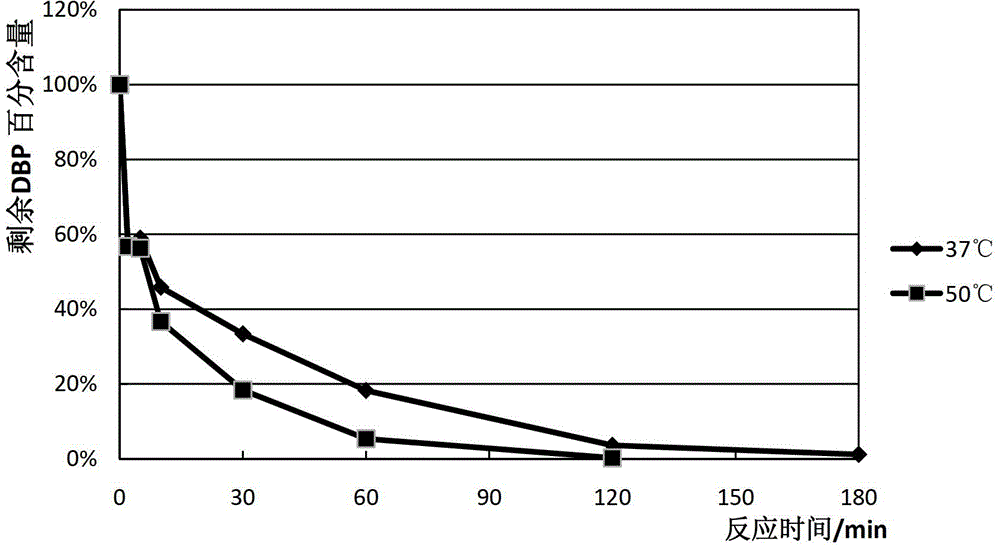
[0130] The obtained crude protease solution and purified protein were tested for protein concentration and enzyme activity, and the specific activity of the enzyme was very high, up to 2600U / mg. Using pNP esters with different chain lengths, measuring the reaction efficiency of the enzyme for different substrates, obtaining the reaction substrate spectrum of the enzyme and the optimal substrate of the enzyme for pNP esters. Detect the resistance of the enzyme to heat, denaturants, organic solvents and metal ions. The enzyme has good thermal stability, has high tolerance to low concentration urea and Tween, and has good resistance to organic solvents and metal ions. general tolerance. Amino acid alignment analysis and conservative sequence analysis showed that the highest similarity between this enzyme and other enzymes was only 52%, and it was determined to be a n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com