Preparation method of biphenyl derivatives
A derivative and biphenyl technology, applied in the field of preparation of biphenyl derivatives, can solve problems such as difficult recovery, unfavorable industrial production, safety accidents, etc., and achieve the effects of avoiding operation process, realizing production cost, and reducing energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
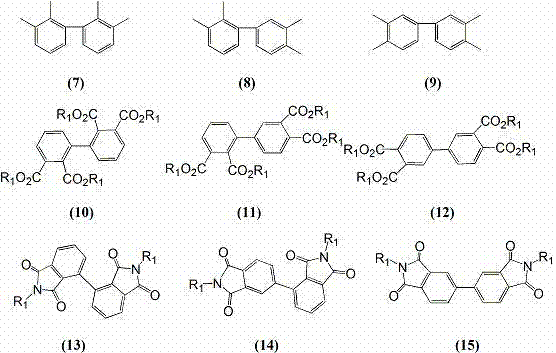
[0035] 4-Bromo-o-xylene (36.6 g, 0.2 moL), zinc powder (13 g, 0.2 moL), anhydrous 2-methyltetrahydrofuran (52 g, 0.6 moL), anhydrous NiCl 2 (0.51 g, 4 mmoL) and triphenylphosphine (2 g, 8 mmoL), mixed in a nitrogen atmosphere, 85 o After C reacted for 8 hours, cooled, filtered off the inorganic matter, and the filtrate recovered 49 g of 2-methyltetrahydrofuran solvent through distillation, and collected 145 ~ 165 by distillation under reduced pressure. o C fraction (pressure 1~3 mmHg). 19.1 g of 3, 3’, 4, 4’-tetramethylbiphenyl was obtained with a yield of 90% and a melting point of 74-75 o c.
Embodiment 2
[0037] 3-Chloro-o-xylene (28 g, 0.2 moL), manganese (1.1 g, 0.2 moL), anhydrous 2-methyl-substituted tetrahydrofuran (43 g, 0.5 moL), anhydrous NiCl 2 (0.51 g, 4 mmoL), bipyridine (1.25 g, 8 mmoL) were mixed in a nitrogen atmosphere, 80 o C reacted for 8 hours, cooled, filtered off inorganic matter, and the filtrate recovered 40 g of 2-methyltetrahydrofuran solvent through distillation, and collected 135 to 155 by distillation under reduced pressure. o Fraction C (pressure 1~3 mmHg), obtained 17.9 g of 2, 2’, 3, 3’-tetramethylbiphenyl with a yield of 85% and a melting point of 115~116 o c.
Embodiment 3
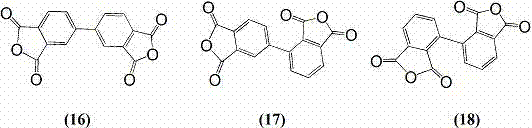
[0039] Dimethyl 4-chlorophthalate (22.8 g, 0.1 moL), zinc powder (6.5 g, 0.1 moL), anhydrous 2,5-dimethyltetrahydrofuran (50 g, 0.5 moL), anhydrous NiBr 2 (0.65 g, 3 mmoL), triphenylphosphine (2.6 g, 10 mmoL), 75 o C reaction for 10 hours, then cooled and filtered to remove inorganic matter, and the filtrate was distilled to recover 46 g of 2,5-dimethyltetrahydrofuran solvent to obtain the crude product of tetramethyl biphenyl tetracarboxylate, which was recrystallized from methanol to obtain 17 g of pure product, with a yield of 88 %. Add the obtained tetramethyl biphenyl tetracarboxylate into a mixture of 100 mL of water and 10 mL of concentrated sulfuric acid, heat to reflux for 24 hours, cool and filter, collect the precipitate, and o C was heated in three stages for 15 hours to obtain 12.5 g of 3, 3', 4, 4'-biphenyltetracarboxylic dianhydride, with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com