Core-shell magnetic poly(m-phenylene diamine) nano-particle, preparation method and application thereof
A poly-m-phenylenediamine and nanoparticle technology, which is applied in other chemical processes, chemical instruments and methods, alkali metal oxides/hydroxides, etc. Uniform loading of components, destruction of the lattice structure of magnetic particles, etc., to achieve the effects of controllable product morphology, easy solid-liquid separation, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
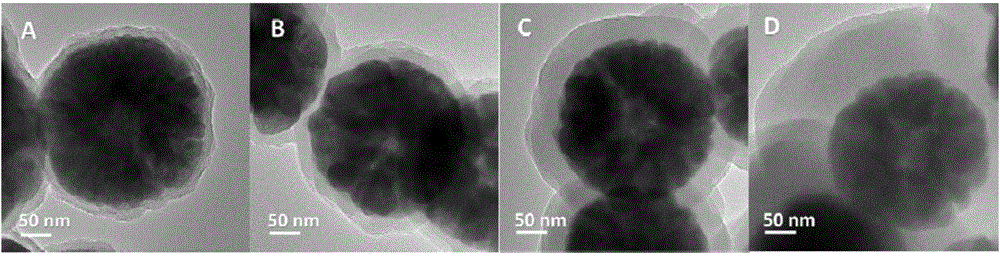
[0036] Take Fe 3 o 4 Add 0.1g into a 150mL Erlenmeyer flask, add 50mL deionized water, and ultrasonically disperse for 10min. Weigh 0.025g of mPD (m-phenylenediamine monomer) and dissolve it in 10mL of deionized water. After dissolving, add ferric oxide dispersion and shake it in an ice-water bath to mix evenly. Weigh 0.055g of sodium persulfate oxidant and dissolve it in 10mL of deionized water, fully dissolve, add ferric oxide and mPD mixture, and continue shaking in ice-water bath for 5h. Take out the solution, magnetically separate, wash with water 3-4 times until the supernatant is colorless, and wash with ethanol twice. The washed sample was dried at 60°C, weighed, and collected. The sample mass was 0.1203g. The samples were used for TEM characterization as figure 1 As shown in A.
Embodiment 2
[0038] Take Fe 3 o 4Add 0.1g into a 150mL Erlenmeyer flask, add 50mL deionized water, and ultrasonically disperse for 10min. Weigh 0.05gmPD and dissolve it in 10mL deionized water. After dissolving, add ferric oxide dispersion, shake in ice water bath to mix evenly. Weigh 0.11g of sodium persulfate oxidant and dissolve it in 10mL of deionized water, fully dissolve, add ferric oxide and mPD mixture, and continue shaking in an ice-water bath for 5h. Take out the solution, magnetically separate, wash with water 3-4 times until the supernatant is colorless, and wash with ethanol twice. The washed sample was dried at 60° C., weighed, and collected. The sample mass was 0.1254 g. The samples were used for TEM characterization as figure 1 Shown in B.
Embodiment 3
[0040] Take Fe 3 o 4 Add 0.1g into a 150mL Erlenmeyer flask, add 50mL deionized water, and ultrasonically disperse for 10min. Weigh 0.1g mPD and dissolve it in 10mL deionized water. After dissolving, add ferric oxide dispersion, and shake it in an ice-water bath to mix it evenly. Weigh 0.22g of sodium persulfate oxidant and dissolve it in 10mL of deionized water, fully dissolve, add ferric oxide and mPD mixture, and continue shaking in ice-water bath for 5h. Take out the solution, magnetically separate, wash with water 3-4 times until the supernatant is colorless, and wash with ethanol twice. The washed sample was dried at 60°C, weighed, and collected. The sample mass was 0.1406g. The samples were used for TEM characterization as figure 1 C shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com