Applications of glycosyltransferase and mutants thereof to synthesis of ginsenoside Rh2
A technology of glycosyltransferase and ginsenoside, which is applied in the field of biopharmaceuticals to achieve the effect of high output, low consumption and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Screening of glycosyltransferases for the synthesis of ginsenoside Rh2 by transglycosylation of protopanaxadiol
[0048] With the help of Rxnfinder, a search tool based on compounds and chemical reactions, combined with database resources such as PIR and NCBI, and based on the principles of substrate similarity and catalytic reaction type, some glycosyltransferase genes that may catalyze the glycosylation of protopanaxadiol were screened. They are UGT51 derived from Saccharomyces cerevisiae, OleD derived from Streptomyces antibiotics and SGT2 derived from potato. Among them, UGT51 derived from Saccharomyces cerevisiae was obtained by cloning, OleD derived from Streptomyces antibiotics and SGT2 derived from potato were obtained by whole gene synthesis. The full-length ORFs of OleD and SGT2 genes were cloned into pET28a(+) plasmid with NdeI and XhoI restriction sites.
[0049] The genome of Saccharomyces cerevisiae S288c was extracted using a fungal genome extr...
Embodiment 2
[0061] Example 2 Separation, purification and structural identification of glycosylation products
[0062]Prepare 100 mL of reaction solution according to the ratio in Example 1. Under the condition of 30°C, the reaction was carried out for 48 hours. The reaction was terminated by adding the same volume of n-butanol. The upper organic phase was dried by rotary evaporation, purified by silica gel column, the eluent was chloroform:methanol=85:15, and each 5mL aliquot was collected, and the collected samples were analyzed by TLC (conditions were the same as above) to obtain protopanaxadiol Glycosylation product fraction, purity <90%.
[0063] The collected fractions were further purified using a Sep-Pak tC18 column (Waters) with water (A) and acetonitrile (B) as eluents, using a gradient elution (20%B, 40%B, 50%B, 60%B) B, 65% B, 70% B, 75% B, 80% B, 85% B, 90% B, 100% B), at 70% and 75% acetonitrile elution, to obtain protopanaxadiol glycosylation Product fraction, the purit...
Embodiment 3
[0064] Example 3 In vitro enzymatic transformation of wild-type UGT glycosyltransferase to synthesize ginsenoside Rh2
[0065] Option One
[0066] According to Example 1, the crude enzyme solution of UGT51 glycosyltransferase was obtained.
[0067] The reaction solution was prepared with dimethyl sulfoxide (DMSO), protopanaxadiol, uridine diphosphate glucose (UDPG), and Tris-HCl buffer. The proportion of organic solvent DMSO in the reaction solution was 10% (v / v). Panaxadiol was 0.5g / L, the molar concentration of Tris-HCl buffer was 50mmol / L, pH was 7.5, and the ratio of UDPG to protopanaxadiol was 10:1 (w / w). The crude UGT51 glycosyltransferase enzyme solution was added to the reaction solution at a ratio of 70% (v / v), and the reaction was carried out for 48 hours at 30° C. and 180 rpm.
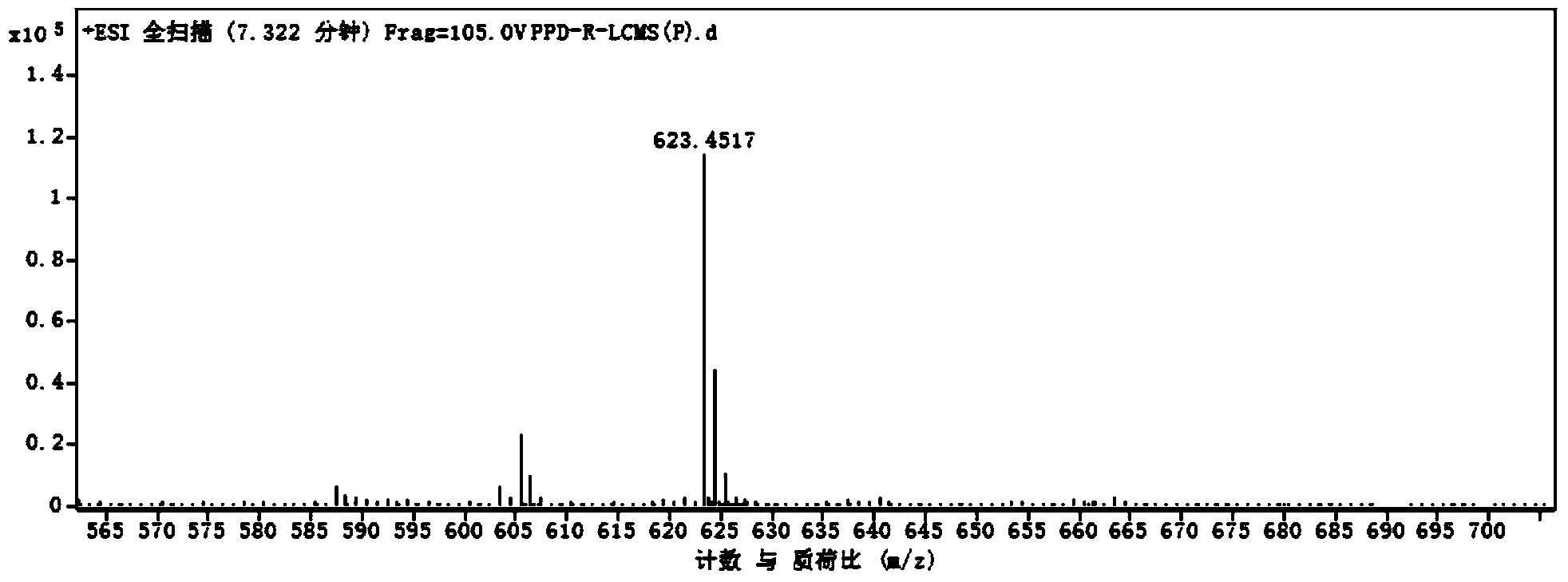
[0068] The same volume of n-butanol was added to terminate the reaction. The extracted organic phase was vacuum-dried and dissolved in methanol. The HPLC analysis and quantification were c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com