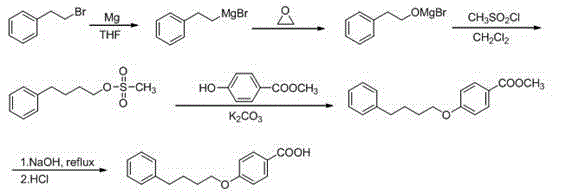
Novel synthesis route of pranlukast intermediate 4-(4-phenylbutoxyl)benzoic acid
A technology of phenyl butoxy sulfonate and phenyl butoxy, which is applied in the field of new synthetic route of pranlukast intermediate 4-benzoic acid, can solve the problem of unexplained starting material 4-chloro-1- Problems such as the preparation method of butanol, and achieve the effects of low cost, easy availability of raw materials, and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] a. Synthesis of 4-phenylbutoxysulfonate
[0044] Add magnesium powder (2.64 g, 0.11 mol), a small grain of iodine and partially dried tetrahydrofuran (total 15 mL, 0.5 mol) and β-bromophenylethane (a total of 18.5 g, 0.1 mol) mixed solution, the reaction was initiated at 25 oC. After the reaction is initiated, slowly add the remaining tetrahydrofuran and β-bromophenylethane mixture dropwise under stirring, and control the dropping rate to keep the system slightly boiling. After the dropwise addition, continue heating to reflux for 0.5 h, stop heating, and cool to room temperature. The reaction system was placed in an ice-salt bath, and the temperature was kept at 0 oC. Ethylene oxide (4.4 g, 0.1 mol) was introduced into the reaction solution via catheter. After the feeding was completed, the ice bath was removed, and slowly heated to reflux in a water bath for 1 h, then cooled to room temperature, and the solvent tetrahydrofuran was distilled off to obtain a viscous ...
Embodiment 2
[0051] a. Synthesis of 4-phenylbutoxysulfonate
[0052] Add magnesium powder (2.64 g, 0.11 mol), a small grain of iodine and partially dried tetrahydrofuran (total 15 mL, 0.5 mol) and β-bromophenylethane (a total of 18.5 g, 0.1 mol) mixed solution, the reaction was initiated at 25 oC. After the reaction is initiated, slowly add the remaining tetrahydrofuran and β-bromophenylethane mixture dropwise under stirring, and control the dropping rate to keep the system slightly boiling. After the dropwise addition, continue heating to reflux for 0.5 h, then stop heating, and then lower to room temperature. The reaction system was placed in an ice-salt bath, the temperature was maintained at 3 oC, and ethylene oxide (4.4 g, 0.1 mol) was introduced into the reaction solution through a catheter. After the feeding was completed, the ice bath was removed, and slowly heated to reflux in a water bath for 1 h, then cooled to room temperature, and the solvent tetrahydrofuran was distilled of...
Embodiment 3
[0059] a. Synthesis of 4-phenylbutoxysulfonate
[0060] Add magnesium powder (2.64 g, 0.11 mol), a small grain of iodine and partially dried tetrahydrofuran (total 15 mL, 0.5 mol) and β-bromophenylethane (a total of 18.5 g, 0.1 mol) mixed solution, the reaction was initiated at 25 oC. After the reaction is initiated, slowly add the remaining tetrahydrofuran and β-bromophenylethane mixture dropwise under stirring, and control the dropping rate to keep the system slightly boiling. After the dropwise addition, continue heating to reflux for 0.5 h, then stop heating, and then lower to room temperature. The reaction system was placed in an ice-salt bath at a temperature of 5 oC. Ethylene oxide (4.84 g, 0.11 mol) was introduced into the reaction solution through a catheter. After the feeding was completed, remove the ice bath, slowly heat and reflux in a water bath for 1 h, then cool to room temperature, distill off the solvent tetrahydrofuran, and the reaction system is viscous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com