Application of carbon dot as anti-tumor medicine carrier
An anti-tumor drug, carbon dot technology, applied in the field of nanomedicine, can solve the problems of protein non-specific adsorption, poor drug loading stability, increased drug side effects, etc., and achieves high drug loading rate, good water solubility, and high quantum yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
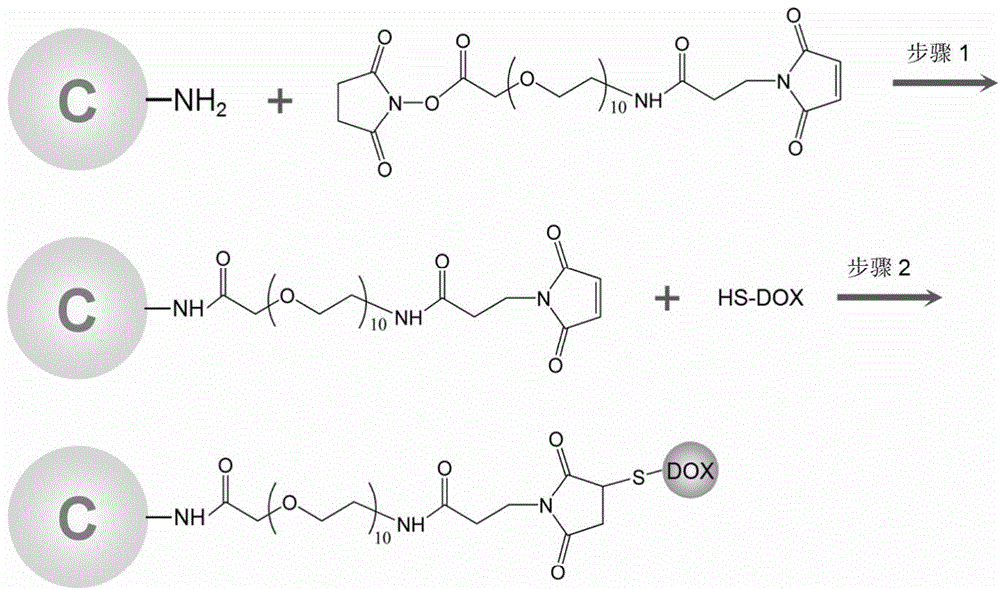
[0037] The preparation of thiolated doxorubicin comprises the following steps:
[0038] (1) Dissolve doxorubicin in a solution (pH=8.0) of dimethyl sulfoxide (DMSO) and phosphate buffer (PBS), and add Traut in the amount of 3 times the substance of doxorubicin ’ s reagent;
[0039] (2) React at room temperature for 1 hour to obtain thiolated doxorubicin.
Embodiment 2
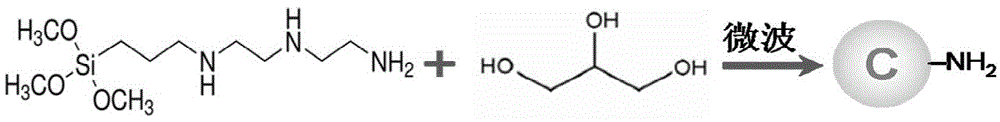
[0041] Preparation of AEEA carbon dots, see figure 1 , including the following steps:
[0042] (1) Into the glycerol, feed nitrogen for 5 minutes to remove the oxygen in the glycerol;
[0043] (2) Add 3-[2-(2-aminoethylamino)ethylamino]propyltrimethoxysilane (AEEA) to glycerol to make its volume fraction 9.1% (5-30%), in a closed state Stir for more than 5 minutes to form a carbon dot precursor solution;
[0044] (3) React in a microwave reactor at 160°C for 15 minutes;
[0045] (4) Centrifuge to remove the precipitate generated, and leave the supernatant;
[0046] (5) Use a dialysis bag with a molecular weight of 1000 to dialyze the supernatant in ultrapure water for more than 48 hours (change the water every 6 hours) to obtain a pure carbon dot solution.
Embodiment 3
[0048] The preparation of aminated carbon dots is similar to that of Example 2, except that in step (3) the microwave reactor was reacted at 180°C for 5 minutes, and the 3-[2-(2-aminoethylamino)ethylamino]propane The volume fraction of trimethoxysilane (AEEA) is 5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com