Construction, expression and purification and functional identification of tissue plasminogen activator
A technology of plasminogen and activator, applied in the field of identification of the protein function, can solve the problems of intracranial hemorrhage, weak targeting, increase production cost, etc., and achieve the effect of improving the weak targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
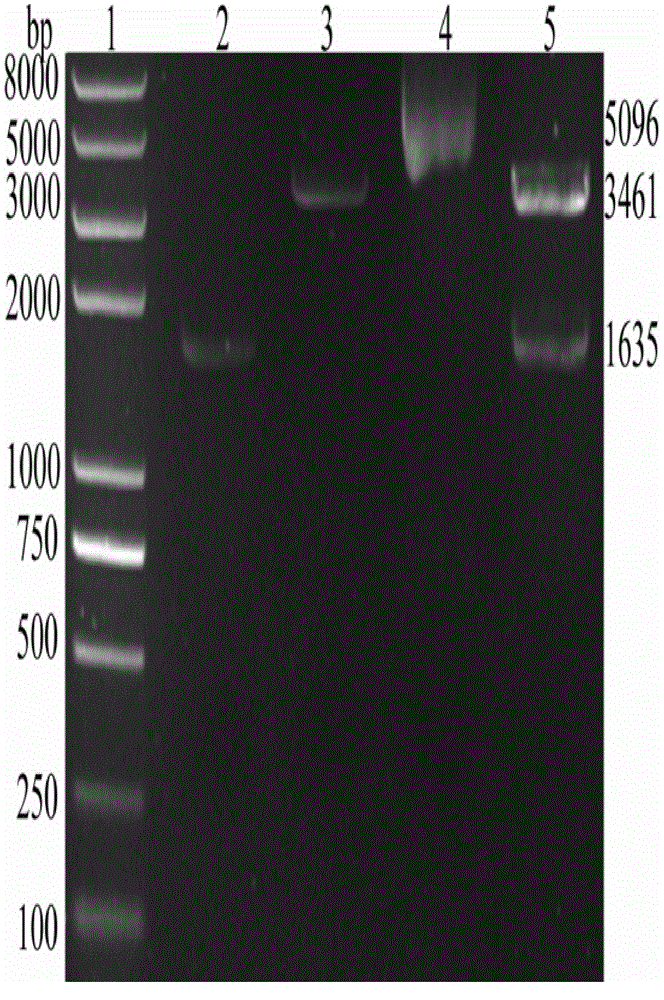
[0018] Example 1 Construction of recombinant tissue-type plasminogen activator gene
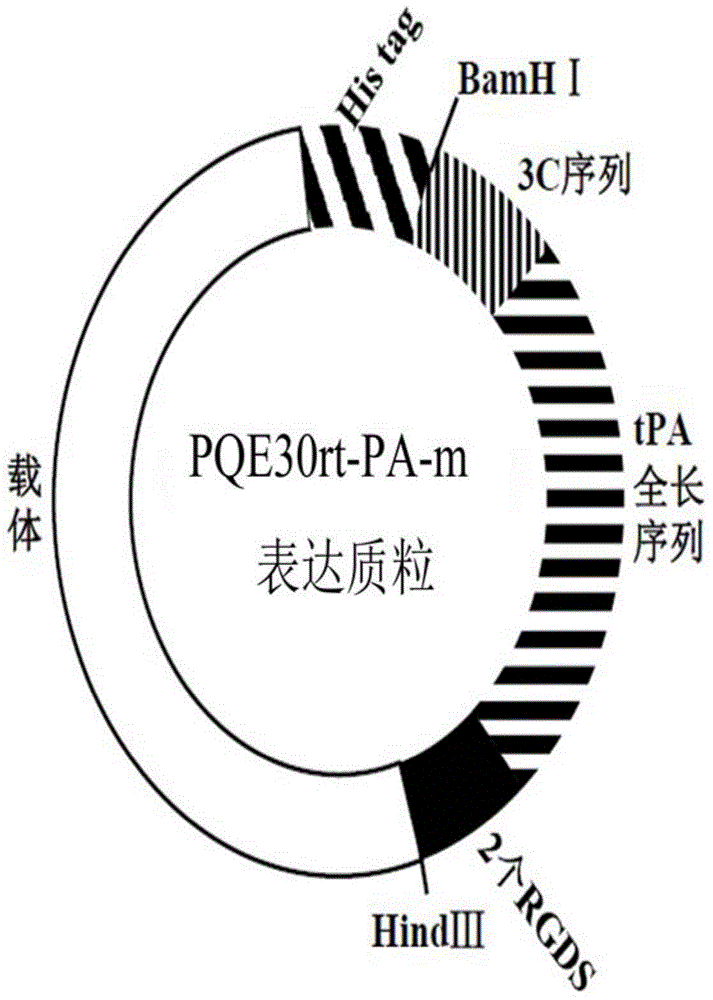
[0019] The schematic diagram of the construction of the recombinant tissue plasminogen activator mutant is shown in figure 1 , the nucleotide and amino acid sequences are respectively shown in the sequence listing SEQ ID NO.1 and SEQ ID NO.2.
[0020] 1. Construct EST-tPA plasmid (a plasmid containing the full length of human tPA gene):
[0021] SEQ ID NO.3 (a total of 1584 bases in the nucleotide sequence):
[0022] SEQ ID NO.4 (the amino acid sequence has a total of 527 bases):
[0023] 2. Obtaining of recombinant rt-PA-m:
[0024] Design primers:
[0025] The upstream primer is: 5'- GGATCC ATG TCTTACCAAGTGATCTGCAGAGAT-3', BamHI cutting GGATCC, followed by the initiation codon ATG, the underlined part: CTGGAAGTTCTGTTCCAGGGGCCC is the 3C enzyme gene sequence, followed by the nitrogen terminal initiation sequence of the original tPA.
[0026] The downstream primer is: 5'- AAGCTT TT...
Embodiment 2
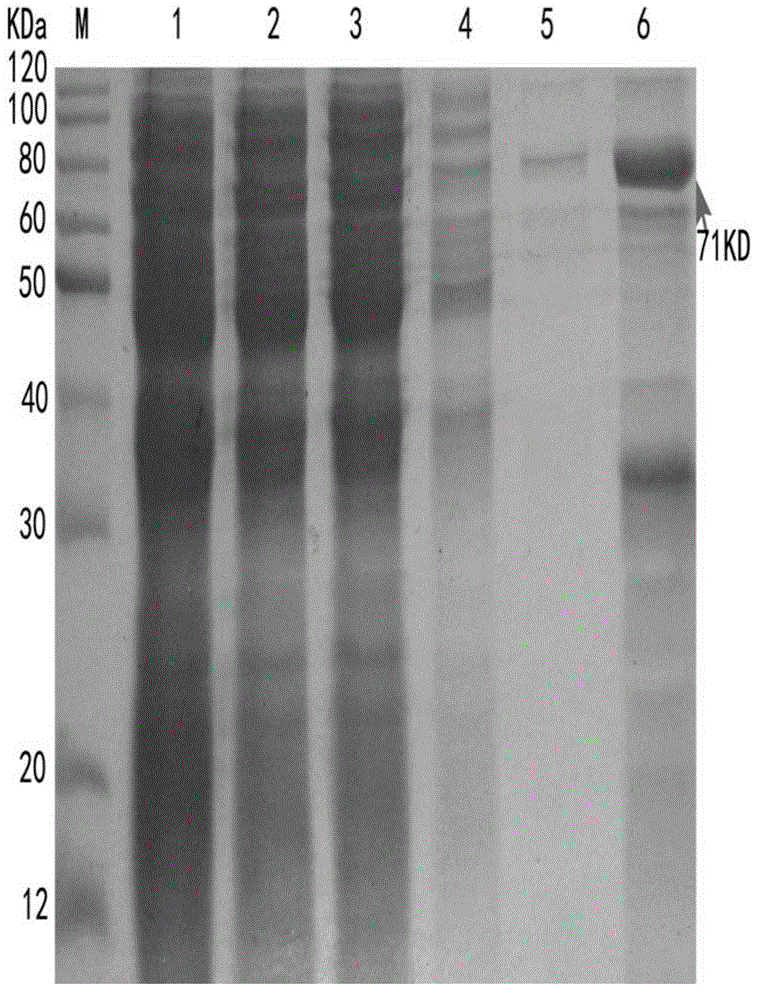
[0051] Example 2 Self-induced supernatant expression of expression plasmid in E. coli and purification of His-3C-tPA-RGDS protein
[0052] 1. Transformation of expression bacteria
[0053] The specific method is as follows: Take a tube of 50ul BL21(DE3) competent cells stored at -80°C and thaw on ice for 15 minutes; add 10 μl of the ligation product (His-3C-tPA-RGDS) to BL21(DE3) competent cells Mix gently, place on ice for 15 minutes; heat shock at 42°C for 1 minute, quickly place on ice, and ice bath for 15 minutes; add 100 μl non-resistant LB liquid medium, recover at 37°C at low speed (160rpm) for 45 minutes; Streak inoculation on ampicillin-resistant LB plates, and incubate in a 37°C incubator for 8-16 hours.
[0054] Transform the positive recombinant plasmid with correct sequencing into Escherichia coli BL21 (DE3) strain (commercially available, New England Biolabs (NEB) company article number: C2527H), streak inoculated on the ampicillin-resistant LB plate, and incuba...
Embodiment 3
[0071] Embodiment 3 tPA-RGDS thrombolytic activity identification
[0072] Preparation of fibrin plate; weigh 1g of agarose, add it to 100mL Tris-HCl buffer solution (composition: 50mM Tris-HCl, pH7.5, 100mM NaCl), sterilize by autoclaving at 115℃×10min, take 30ml of agar liquid Configure a plate, and when the temperature of the melted agar liquid drops to 37-42°C, add 1000 μL of 25 mg / mL fibrinogen mother solution and 1000 mM CaCl to it in turn. 2 300uL of mother solution, 500μL of 6.0IU / mL plasminogen mother solution, then incubate and shake at 37℃ for 30sec, then add 30μL of 100U / ml thrombin mother solution, incubate and shake at 37℃ for about 60-80sec, then turbidity can be seen; pour it into a plate immediately After 2 minutes, place the slightly solidified plate in a refrigerator at 4°C for about 10-20 minutes before use. ) Punch holes with a hole punch with a diameter of 3mm. The fusion protein and urokinase standard were diluted and added to each well, and incubated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com