Preparation method of graphite type carbon nitride photocatalytic material
A catalytic material, graphite-based technology, used in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as product agglomeration, low specific surface area, and damage to semiconductor characteristics, and achieve visible light catalytic efficiency. High, broad application prospects, the effect of reducing the compound rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A method for preparing a graphite-type carbon nitride photocatalytic material, the preparation method comprising the following steps:
[0023] (1) Thoroughly grind and mix barbituric acid, dicyandiamide, lithium chloride and potassium chloride.
[0024] (2) The mixture is placed in a calcination vessel and calcined under a nitrogen atmosphere.
[0025] (3) Grinding the calcined product, ultrasonication, centrifugation, and drying to obtain the g-C 3 N 4 catalyst.
[0026] The molar ratio of barbituric acid and dipolycyanamide described in step (1) is between 0.04~0.22; The mass ratio of described lithium chloride and potassium chloride is 9:11; The described lithium chloride and chlorine The mass ratio of the total mass of potassium chloride to dipolycyanamide is 5:1.
[0027] The calcination process described in step (2) is: heating to 400°C at a rate of 7°C / min, holding for 6 hours, continuing to heat at the same heating rate to 570°C, and holding for 12 hours...
Embodiment 1
[0031] Example 1: Block g-C 3 N 4 Catalyst preparation
[0032] Take 2g of dicyandiamide and put it into a corundum ark, place it in the middle of a tube furnace, heat it up to 550°C at a heating rate of 2.5°C / min, and keep it at 550°C for 4 hours. The whole process is carried out under the protection of nitrogen. After natural cooling, it was taken out and ground with a mortar to obtain a yellow powder sample.
Embodiment 2
[0033] Embodiment 2: layered modified g-C 3 N 4 preparation of
[0034] Take 2g of dicyandiamide, 0.29g of barbituric acid (the molar ratio of barbituric acid to dicyandiamide is 0.10), 4.5g of lithium chloride, and 5.5g of potassium chloride, and grind them in a mortar for 20 minutes ; Place the mixture in the middle of the tube furnace, heat it to 400°C at a rate of 7°C / min, and keep it at 400°C for 6 hours; continue to heat it to 570°C at a rate of 7°C / min, and keep it at 570°C After 12 hours, the whole process was carried out under the protection of nitrogen. After natural cooling, the block was taken out, ground for 20 minutes, ultrasonicated for 1 hour, rinsed with boiling water at 100°C for 3 times, and centrifuged at a rate of 10,000 rpm for 5 minutes, take out the suspension, and dry it at 80°C for 2 days to obtain the final sample.
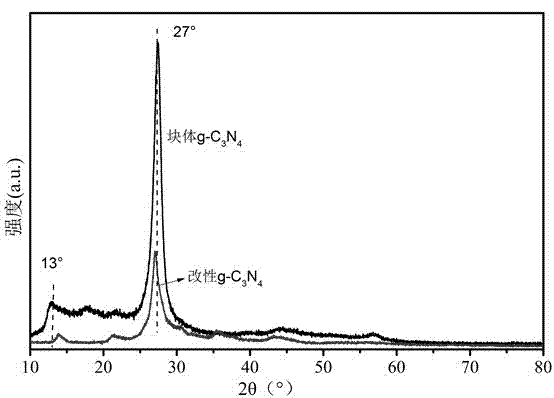
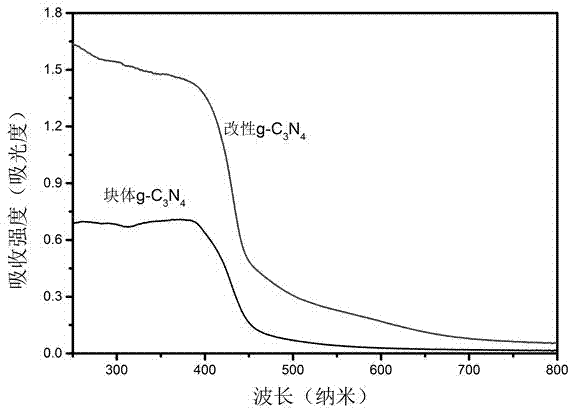
[0035] The catalyst prepared by the method of embodiment 1 and 2, after X-ray diffractometer scanning, see figure 1 , the synt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com