Ferric ion fluorescent probe compound as well as preparation method and application thereof
A technology of fluorescent probes and compounds, applied in the field of fluorescent probes, can solve the problems such as the failure to apply detection and imaging of fluorescent probes, the non-linearity of the response of fluorescent probes, and the inability to quantitatively test the concentration of iron ions, etc. Beneficial for observation and detection, good selectivity and sensitivity, and good promotion prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the preparation method of ferric ion fluorescent probe compound, route is as follows:
[0047]
[0048] (a) Take 2.4 grams of rhodamine B, dissolve it in 20 milliliters of ethanol, add 2.5 milliliters of anhydrous ethylenediamine, stir and reflux for 15 hours, until the red color of the solution fades. The solvent was evaporated to dryness, 20 ml of water was added, extracted with dichloromethane, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain the rhodamine amide product shown in formula II.
[0049] (b) Under nitrogen protection, 2.5 g of binaphthol was dissolved in 100 ml of tetrahydrofuran, the solution was cooled to 0° C., and 0.88 g of sodium hydride was slowly added, and stirred for 15 minutes. 1.65 ml of 98% chloromethyl methyl ether was slowly added, the mixture was warmed to room temperature and reacted for 4 hours, then quenched by adding water. It was extracted with ethyl acetate, dried over anhydrous sodium su...
Embodiment 2
[0054] Embodiment 2, fluorescence experiment
[0055] The fluorescent probe compound prepared in Example 1 was dissolved in an aqueous solution containing 30% tetrahydrofuran, and the pH was adjusted to 7.0 with HEPES buffer solution (Zhangjiagang Changhua Chemical Co., Ltd.); the fluorescent probe solution was obtained for future use.
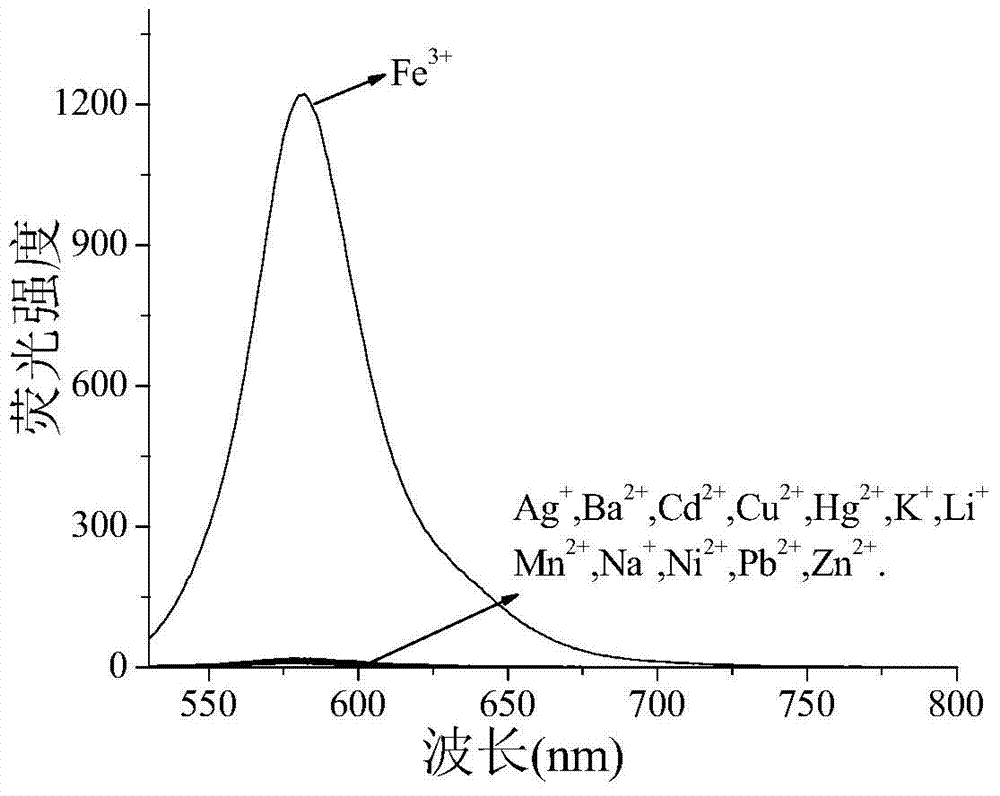
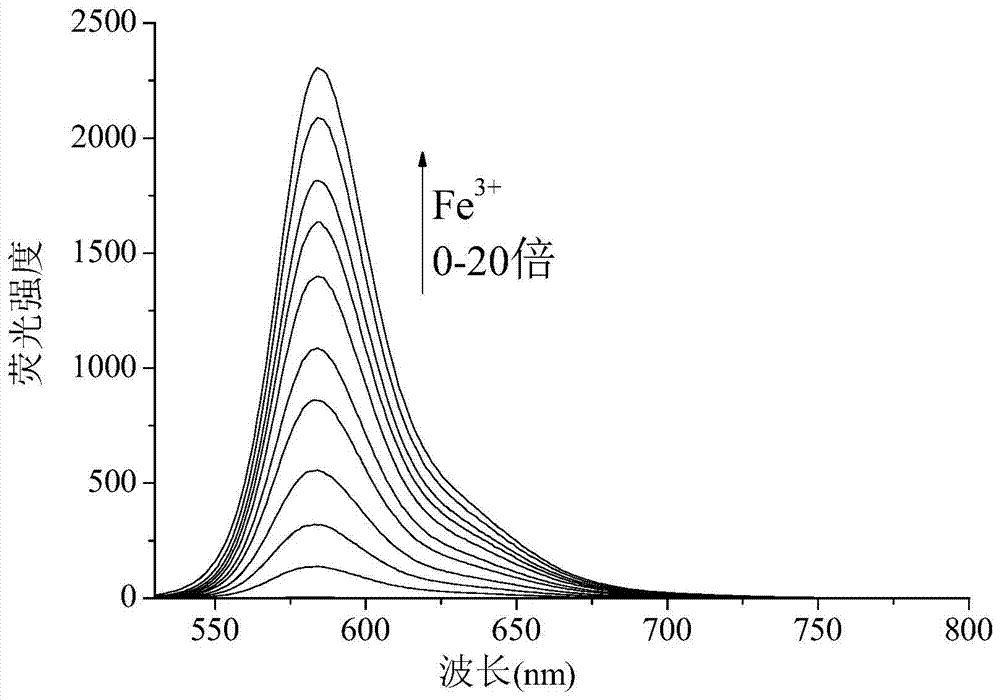
[0056] 1. Take the fluorescent probe solution, divide it into 13 groups, each group (bottle) 10 ml, add the solution containing Fe 3+ 、Cd 2+、 Pb 2+ , Hg 2+ 、Na 2+ 、Ba 2+ 、K + , Mn 2+ , Li + 、Cu 2+ , Zn 2+ 、Ag + 、Ni 2+ solution, so that the concentration of the probe compound contained in each group of solutions is 50 μM, and the metal ion concentration is 500 μM, so that the molar ratio of the metal ion to the probe compound is 10:1; the excitation wavelength is 510 nm, and its fluorescence intensity is tested by a fluorescence photometer ,Such as figure 1 As shown, the results show that: the solution added with iron ions has a st...
Embodiment 3
[0058] Embodiment 3: Fluorescent probes of the present invention are used in cell imaging experiments
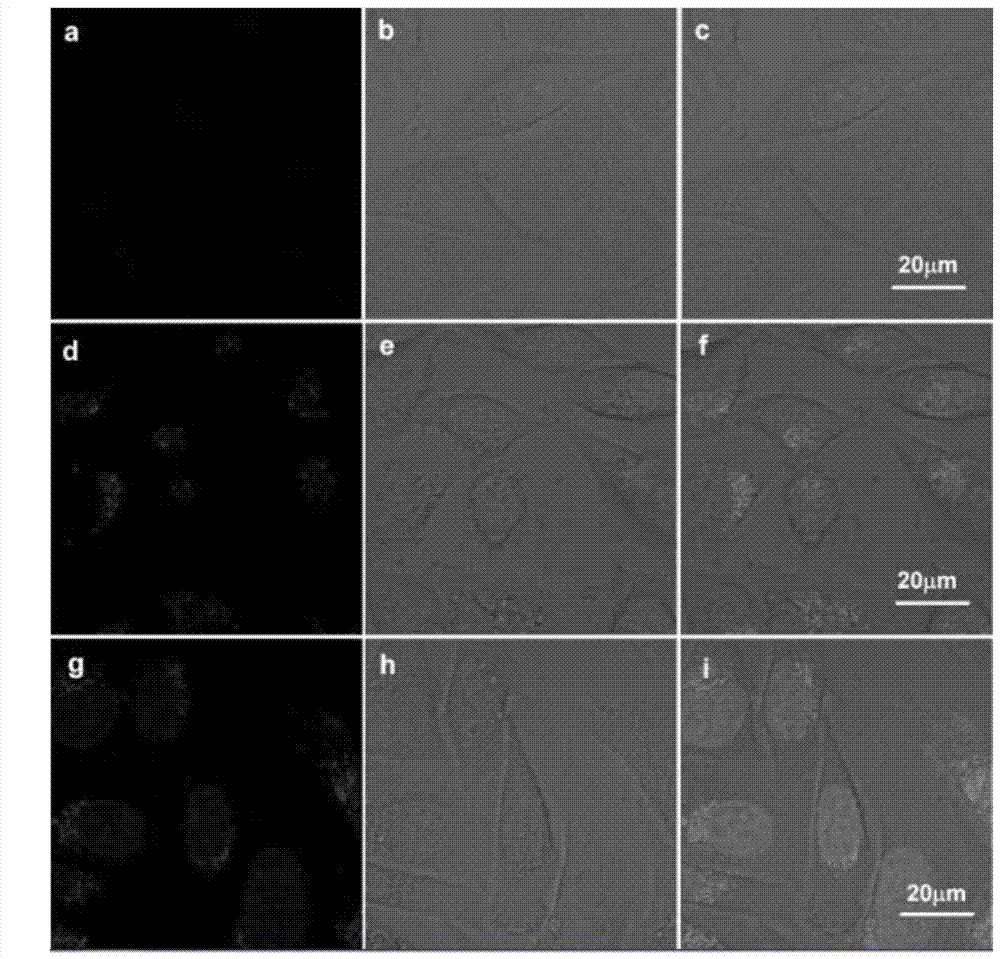
[0059] The fluorescent probe compound prepared in Example 1 was used for cell imaging, and the obtained cell micrograph is shown in Figure 3. In the figure, a, b, c are images of HeLa cells cultured and treated with this fluorescent probe, and it can be seen that there is no obvious fluorescence; d, e, f are HeLa cells cultured and treated with ferric ions In the figure, there is no fluorescence; g, h, and i are HeLa cells treated with ferric ions, and then added this fluorescent probe compound for culture, and obvious fluorescence imaging can be seen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com