Method for purifying pneumococcal capsular polysaccharide
A technology of Streptococcus pneumoniae and capsular polysaccharide, applied in the fields of biology and vaccines, can solve the problems of increasing operation steps and time, increasing the risk of cross-contamination, loss of immunogenicity of polysaccharides, etc. The effect of improving the quality of pneumococcal polysaccharide vaccine and improving the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
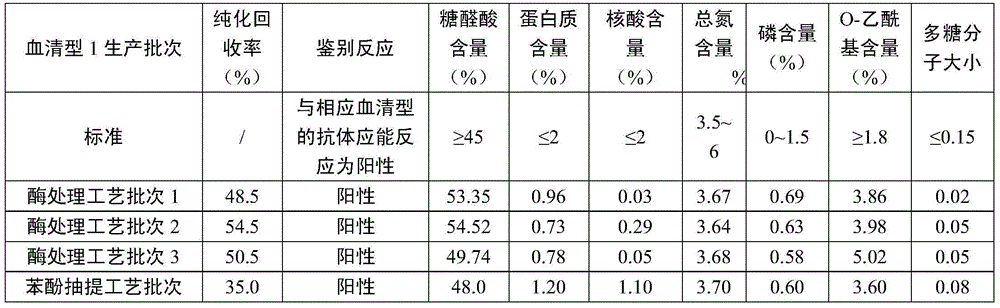
[0039] Streptococcus pneumoniae type 1 was fermented in a bioreactor; grown to the late logarithmic growth phase, sterilized and lysed with sodium deoxycholate at a final concentration of 0.1%. Centrifuge at 9000g for 50 minutes, and carry out ultrafiltration and concentration of the feed liquid with a membrane bag with a molecular weight cut-off of 100KD. After the fermentation broth volume is concentrated by 8 times, sodium acetate is added to make the final mass percentage concentration 7%, and pre-cooled 1- Propanol to make the final concentration of the volume percentage to 20%, settling at 4°C for 8 hours, centrifuging to recover the supernatant, replacing the 1-propanol and salt ions in the alcohol supernatant by buffer ultrafiltration, and obtaining Streptococcus pneumoniae Type 1 alcohol precipitation supernatant ultrafiltrate. Adjust the pH of the supernatant ultrafiltrate of alcohol precipitation to 8.5, add MgCl 2 Make the final concentration to 2mM, add Benzonase...
Embodiment 2
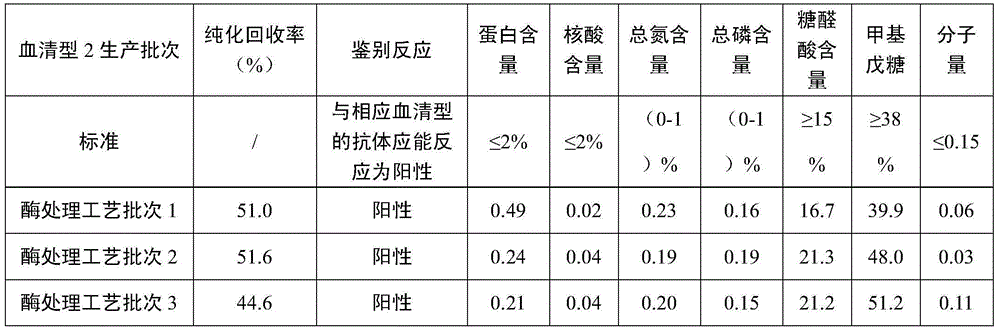
[0046] Streptococcus pneumoniae type 2 is fermented in a bioreactor; grow to the late logarithmic growth phase and sterilize with a lysing agent and lyse the bacteria. After being centrifuged at 9000g for 50 minutes and concentrated by ultrafiltration of a membrane bag with a molecular weight cut-off of 100KD, the volume of the fermented liquid was concentrated 8 times, and then sodium acetate was added to make the final mass percentage concentration 5%, and 2-propanol that had been cooled in advance was added to make it The final concentration of the volume percentage is 25%, let it stand for 22 hours at 4°C, centrifuge to recover the supernatant, replace the 2-propanol and salt ions in the alcohol precipitation supernatant with buffer ultrafiltration, and obtain the alcohol precipitation supernatant ultrafiltrate . Adjust the pH to 8.0, add MgCl 2 Make the final concentration to 3mM, add Benzonase nuclease to make the final concentration to 25U / ml, stir the reaction at 37°C...
Embodiment 3
[0054]Streptococcus pneumoniae type 5 is fermented in a bioreactor; grow to the late logarithmic growth phase and use a lysing agent to sterilize and lyse the bacteria. After centrifuging at 9000g for 50 minutes and concentration by ultrafiltration, the volume of the fermented liquid was concentrated 8 times, then sodium acetate was added to make the final mass percentage concentration 4%, and pre-cooled ethanol was added to make the final volume percentage concentration 12.5%, at 4°C Set aside to settle for 20 hours, centrifuge or filter to recover the supernatant, replace ethanol and salt ions in the alcohol precipitation supernatant with buffer ultrafiltration, and obtain the alcohol precipitation supernatant ultrafiltrate. Adjust the pH to 7.0 and add MgCl 2 Make the final concentration to 3mM, add Benzonase nuclease to make the final concentration to 30U / ml, stir and react at 37°C for 6h, the reaction catalyzed by Benzonase nuclease ends; adjust the pH to 7.5, add calcium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com