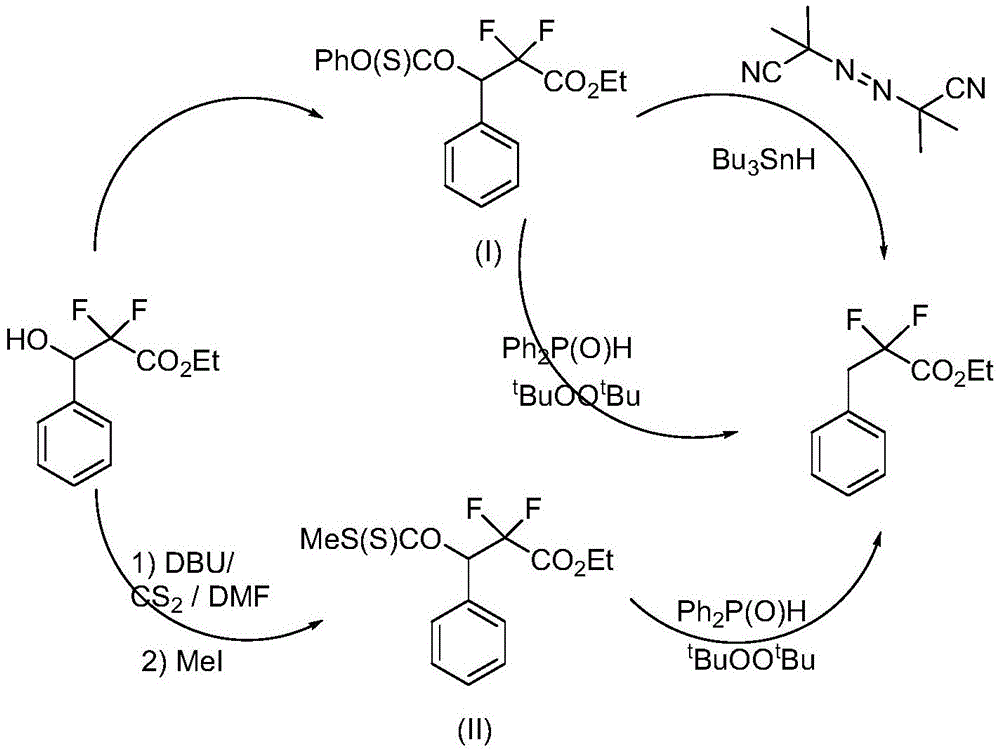
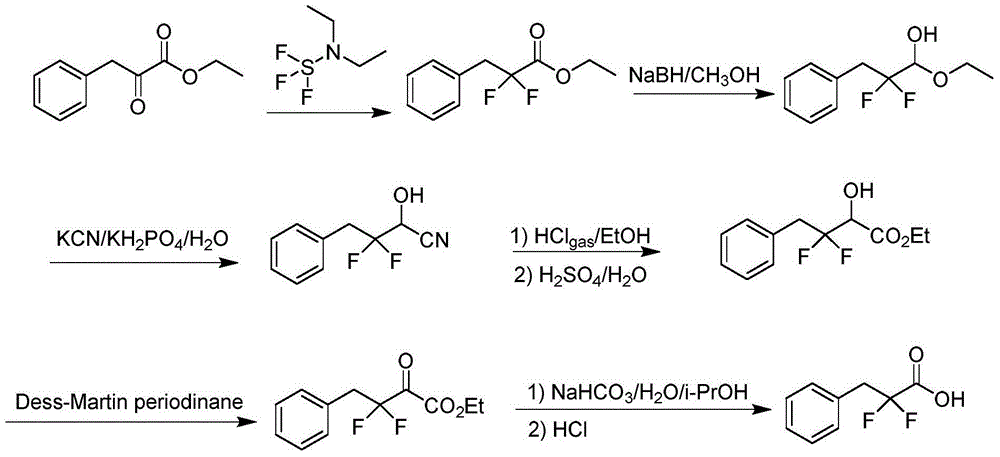
Preparation method of 3-(bromophenyl)-2,2'-difluoropropanoic acid
A technology of difluoropropionic acid and ethyl difluoropropionate, which is applied in the field of preparation of 3--2,2'-difluoropropionic acid, can solve the problems of harsh reaction conditions, difficult industrialized production, complicated operation, etc. The raw materials are easy to obtain, easy to operate, and the raw materials are cheap.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
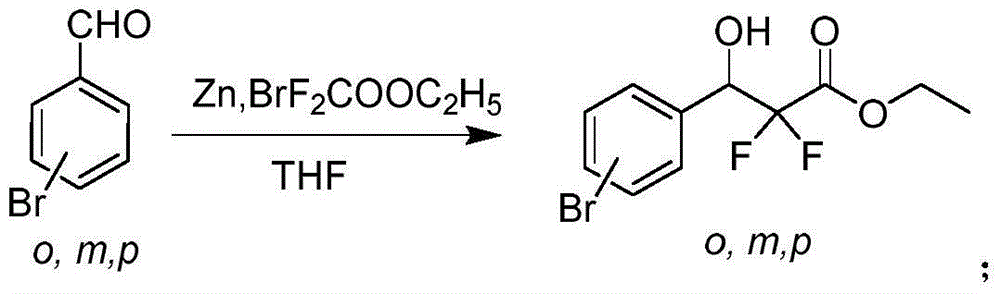
[0024] A kind of preparation method of 3-(4'-bromophenyl)-2,2'-difluoropropionic acid, comprises the steps:
[0025] 1) Under the protection of nitrogen, add 50mL of anhydrous THF and 0.78g of activated zinc powder (12.0mmol) to a 100mL three-necked flask equipped with a magnetic stirrer. Ethyl difluoroacetate (12.0 mmol) was slowly dropped into the mixed solution, keeping the temperature of the reaction system at 55°C. After the dropwise addition, continue stirring for 5-10 minutes, then add 1.85 g of p-bromobenzaldehyde (10.0 mmol) into the above mixture, and heat to reflux for 2 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, and the pH value of the solution was adjusted to 4-6 with dilute hydrochloric acid, extracted 3 times with ethyl acetate (3×100mL), the organic phases were combined, washed with saturated brine, anhydrous sodium sulfate After drying, spin off the solvent under reduced pressure to obtain 1.85 g of yellow oil...
Embodiment 2
[0030] 1) Under nitrogen protection, add 50 mL of anhydrous THF and 0.98 g of activated zinc powder (15.0 mmol) into a 100 mL three-neck flask equipped with a magnetic stirrer. After the addition, the mixed system was heated to 65°C, and 3.05 g of ethyl bromodifluoroacetate (15.0 mmol) was slowly dropped into the mixed solution, keeping the temperature of the reaction system at 65°C. After the dropwise addition was completed, the stirring reaction was continued for 5-10 min, and then 1.85 g of p-bromobenzaldehyde (10.0 mmol) was heated under reflux for 3 h. After the reaction was completed, the reaction liquid was cooled to room temperature, and the pH value of the solution was adjusted to 4-6 with dilute hydrochloric acid, extracted 3 times with ethyl acetate (3×100mL), the organic phases were combined, washed with saturated brine, anhydrous sodium sulfate After drying, spin off the solvent to obtain 2.24 g of yellow oily product 3-(4'-bromophenyl)-2,2'-difluoro-3-hydroxyprop...
Embodiment 3
[0035] 1) Under nitrogen protection, add 50 mL of anhydrous THF and 1.3 g of activated zinc powder (20.0 mmol) into a 100 mL three-neck flask equipped with a magnetic stirrer. After the addition, the mixed system was heated to 70°C, and 3.05 g of ethyl bromodifluoroacetate (15.0 mmol) was slowly dropped into the mixed solution, keeping the temperature of the reaction system at 70°C. After the dropwise addition was completed, the stirring reaction was continued for 5-10 min, and then 1.85 g of p-bromobenzaldehyde (10.0 mmol) was heated under reflux for 5 h. After the reaction was completed, the reaction liquid was cooled to room temperature, and the pH value of the solution was adjusted to 4-6 with dilute hydrochloric acid, extracted 3 times with ethyl acetate (3×100mL), the organic phases were combined, washed with saturated brine, anhydrous sodium sulfate After drying, spin off the solvent to obtain 2.45 g of yellow oily product 3-(4'-bromophenyl)-2,2'-difluoro-3-hydroxypropi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com