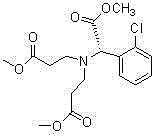
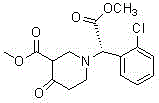
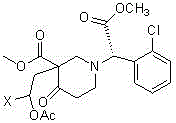
A kind of preparation method of clopidogrel free base
A technology of clopidogrel free base and methyl chlorophenylacetate, which is applied in the field of preparation of clopidogrel free base, can solve the problems of cumbersome preparation, long reaction route, and high price of pyridine, and achieve simple reaction process, simple operation and reliable control, high response selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Preparation of clopidogrel free base (I)
[0048] The reaction scheme is as the aforementioned reaction scheme 3, wherein the 1,2-dihaloethyl acetate of step (3) is selected from 1,2-dibromoethyl acetate, X=Br, and the hydrogen sulfuric acid of step (4) The salt is sodium hydrosulfide nonahydrate (NaHS 9H 2 O).
[0049] The preparation steps are as follows:
[0050] In a 500 ml four-neck flask connected with stirring and a thermometer, add 120 grams of tetrahydrofuran, 20.0 grams (0.1 moles) of (S)-o-chlorophenylglycine methyl ester, 0.1 grams of piperidine, 18.1 grams (0.21 moles) of methyl acrylate , 10-15 ° C and stirred for 4 hours to obtain a solution of the compound of formula II. Then, the solution of formula II compound is added dropwise in the 500 milliliter four-necked flask that 7 gram solid sodium methylate and 50 gram dry tetrahydrofuran are housed, and this four-necked flask is connected with stirring, thermometer, tail gas absorption devi...
Embodiment 2
[0054] Embodiment 2: Preparation of clopidogrel free base (I)
[0055] As described in Example 1, the difference is: 22.0 grams (0.14 moles) of 1,2-dichloroethyl acetate instead of 32.0 grams (0.13 moles) of 1,2-dibromoethyl ethyl acetate in Example 1 Ester, the rest of the steps were the same as in Example 1 to obtain 26.9 grams of clopidogrel free base, with a yield of 83.8% (calculated as (S)-o-chlorophenylglycine methyl ester), and a liquid phase purity of 99.1%.
Embodiment 3
[0056] Embodiment 3: Preparation of clopidogrel free base (I)
[0057] In a 500 ml four-necked flask connected with stirring and a thermometer, add 150 grams of N,N-dimethylformamide, 20.0 grams (0.1 moles) of (S)-o-chlorophenylglycine methyl ester, 0.1 grams of piperidine, 18.1 Gram (0.21 mole) methyl acrylate, 8 to 10 ℃ of stirring reaction 4 hours, obtain the N of formula II compound, N-dimethylformamide solution, the solution that this obtains is added dropwise to 7 grams of solid sodium methylate and 50 grams of N,N-dimethylformamide in a 500 ml four-neck flask (same as Example 1), the dropwise addition process was kept between -10°C and -5°C, and the drop was completed in about 3 hours, after that, stirred at 5°C After 4 hours, the compound of formula III was obtained; after that, 32 grams (0.13 moles) of 1,2-dibromoethyl acetate was added dropwise between 5°C and 10°C for about 1 hour, and then stirred at 20°C for 5 hours , to obtain the compound of formula IV; then be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com