Preparation method of ammonium oxovanadium phosphate crystals
A technology of ammonium vanadyl phosphate and crystals, which is applied in the field of preparation of positive electrode materials, can solve problems such as difficult process control, difficulty in preparing precursors, and cumbersome preparation steps, and achieve the effects of improving conductivity, reducing agglomeration, and having a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Dissolve 0.05mol (5.85g) of ammonium metavanadate, 0.20mol (19.60g) of phosphoric acid, and 0.10mol (19.21g) of citric acid in 100mL of deionized water, and mechanically stir in a water bath at 60°C until Uniform blue solution;
[0034] (2) Regulate pH with ammoniacal liquor (concentration is 20wt%), pH value adjusts pH=7;
[0035] (3) Then transfer it to a polytetrafluoroethylene-lined reactor (Shandong Weihai Self-Control Reactor Co., Ltd., WHFS-10 type reactor) and heat at 280°C for 30 hours, cool to room temperature, take out and filter;
[0036] (4) transfer the filtered product to a vacuum oven at 110° C. for 7 hours to obtain amorphous ammonium vanadyl phosphate powder;
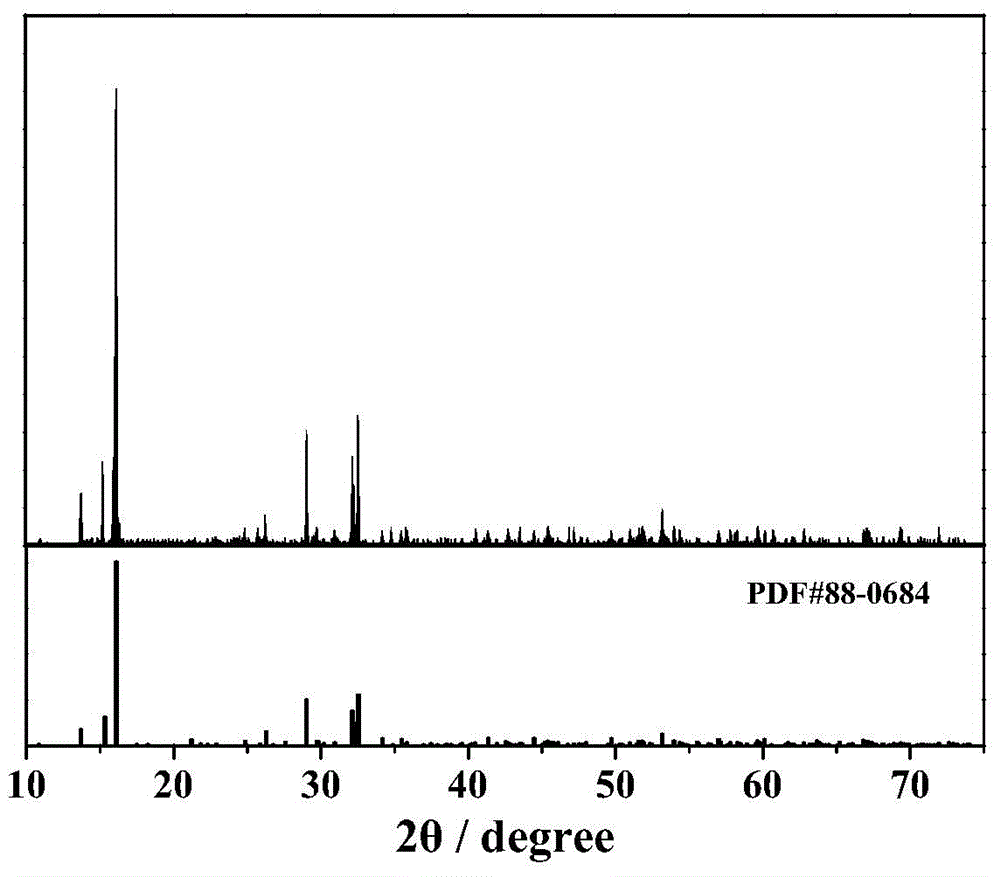
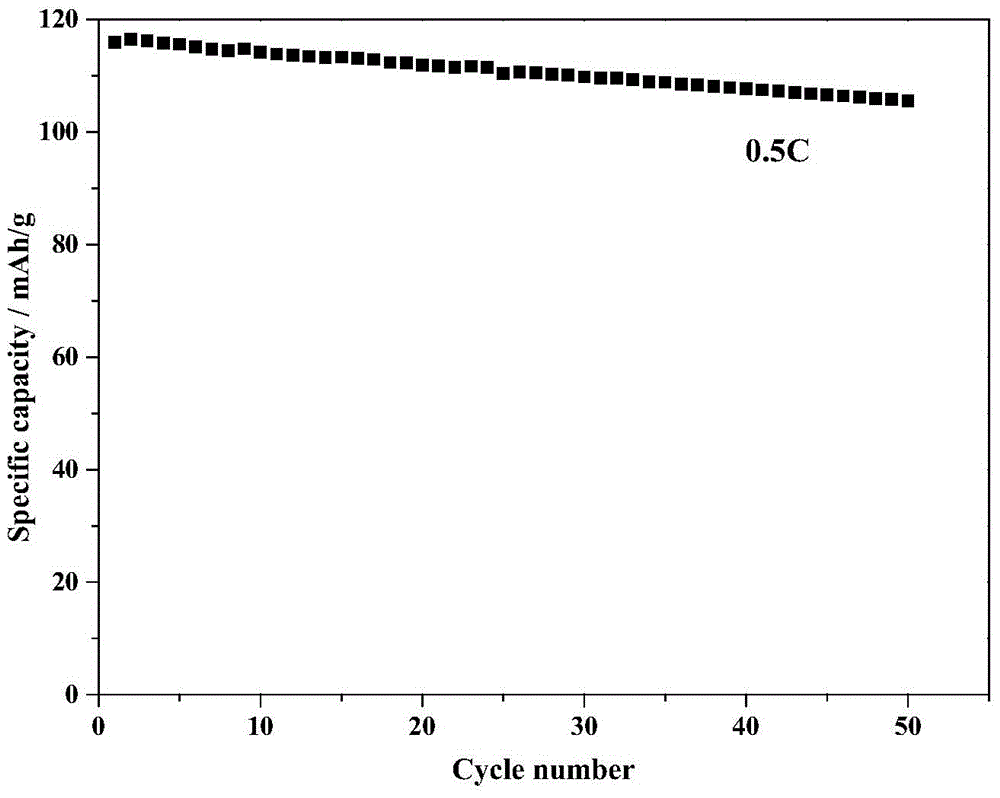
[0037] (5) The amorphous ammonium vanadyl phosphate powder is fully ground in an agate mortar, placed in an air atmosphere muffle furnace, sintered at 550°C for 6h, and then cooled down to room temperature naturally to obtain vanadyl phosphate ammonium crystals. The particle size of the o...
Embodiment 2
[0040] (1) Dissolve 0.05mol (12.25g) of vanadyl oxalate, 0.20mol (26.41g) of diammonium hydrogen phosphate, and 0.50mol (87.00g) of oxalic acid in 500mL of deionized water, and mechanically stir in a water bath at 70°C until A uniform blue solution is formed;
[0041] (2) Regulate pH with ammoniacal liquor (concentration is 20%), pH value adjusts pH=10;
[0042] (3) Then transfer it to a polytetrafluoroethylene-lined reactor at 300°C for heating and reaction for 25 hours, cool to room temperature, take out and filter;
[0043] (4), then transfer the filtered product to a vacuum oven at 110° C. for drying for 9 hours to obtain amorphous ammonium vanadyl phosphate powder;
[0044] (5) The amorphous ammonium vanadyl phosphate powder is fully ground in an agate mortar and evenly placed in a muffle furnace filled with an air atmosphere and sintered at 600 ° C for 5 hours, and then naturally cooled to room temperature to obtain vanadyl phosphate ammonium crystals. The particle si...
Embodiment 3
[0047] (1) Dissolve 0.05mol (9.1g) of vanadium pentoxide, 0.8mol (78.4g) of phosphoric acid, and 0.1mol (17.61g) of ascorbic acid in 1000mL of deionized water, and mechanically stir in a water bath at 80°C until a uniform blue solution;
[0048] (2) Regulate pH with ammoniacal liquor (concentration is 25%), pH value adjusts pH=8;
[0049] (3) Then transfer it to a polytetrafluoroethylene-lined reaction kettle, heat and react at 250°C for 35h, cool to room temperature, take out and filter;
[0050] (4) transfer the filtered product to a vacuum oven at 110° C. for 9 hours to obtain amorphous ammonium vanadyl phosphate powder;
[0051] (5) The amorphous ammonium vanadyl phosphate powder is fully ground in an agate mortar and evenly placed in a muffle furnace filled with an air atmosphere and sintered at 650 ° C for 4 hours, and then naturally cooled to room temperature to obtain vanadyl phosphate ammonium crystals. The particle size of the ammonium vanadyl phosphate material o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com