Saxagliptin medicinal composition and preparation method thereof
A composition and drug technology, which is applied in the direction of drug combination, non-active ingredients of polymer compounds, sugar-coated pills, etc., can solve problems such as the stability of saxagliptin preparations, hygroscopic weight gain of crystalline forms, and high impurity content, and achieve improvement. The effect of in vitro dissolution, simple operation and mature process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
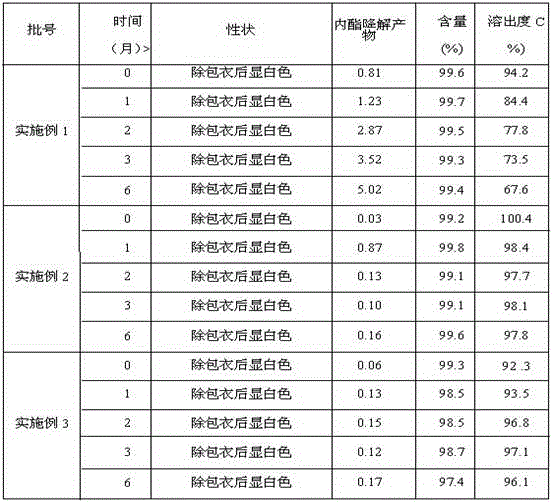
Examples
Embodiment 1
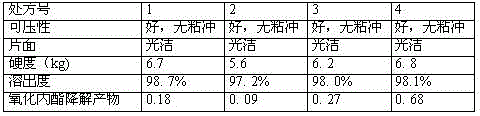
[0105] Embodiment 1 (comparative embodiment)
[0106] Saxagliptin 15-25g
[0107] Croscarmellose Sodium 40-80g
[0108] Microcrystalline cellulose 150-250g
[0109] Lactose 180-360g
[0110] Pregelatinized starch 15-30g
[0112] Appropriate amount of 5% povidone absolute ethanol solution
[0113] Coating powder 100-200g
[0114] Makes 1000 pieces
[0115] The preparation method goes through the following steps:
[0116] 1) Sieve saxagliptin, microcrystalline cellulose, lactose, croscarmellose sodium, pregelatinized starch, and talcum powder separately, and set aside;
[0117] 2) Mix the raw and auxiliary materials evenly and set aside;
[0118] 3) Take 5% povidone anhydrous ethanol solution in 2), mix well, make 50-60 mesh granules, dry below 50°C, granulate, add lubricant, mix evenly, and compress into tablets;
[0119] 4) Put the sample prepared in 3) into the coating pan, coat it, and get it.
Embodiment 2
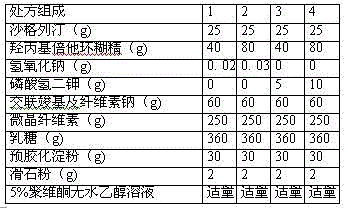
[0121] Composition 1
[0122] Saxagliptin 25g
[0123] Beta cyclodextrin 80g
[0125] Composition 2
[0126] Croscarmellose Sodium 60g
[0127] Microcrystalline cellulose 250g
[0128] Lactose 360g
[0129] Pregelatinized starch 30g
[0130] Talc powder 2g
[0131] Appropriate amount of 5% povidone absolute ethanol solution
[0132] Coating Composition
[0133] Coating powder 200g
[0134] Makes 1000 pieces
[0135] The preparation method goes through the following steps:
[0136] 1) Put saxagliptin, beta cyclodextrin and sodium hydroxide in a mortar, add a small amount of water and grind them into a paste,
[0137] Dried below 50°C, crushed into 150-250 mesh fine powder, set aside;
[0138] 2) Sieve microcrystalline cellulose, lactose, croscarmellose sodium, pregelatinized starch, and talcum powder respectively, and set aside;
[0139] 3) Add the prescribed amount of excipient 2) into 1), mix well, and set aside;
[0140] 4) Take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com