Injectable bone cement and preparation method thereof
A bone cement and injection technology, applied in medical science, prosthesis, etc., can solve problems such as insufficient strength, mismatch between degradation and absorption and bone regeneration rate, and achieve improved injectability, good biocompatibility and degradability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Bone cement composite powders include α-calcium sulfate hemihydrate, tetracalcium phosphate and polyethylene glycol diacrylate powder. At room temperature, weigh 3.996g (99.9%) calcium sulfate hemihydrate and 0.004g (0.1%) polyethylene glycol diacrylate, and mix them uniformly; measure 2 mL of 0.01% mercaptolated hyaluronic acid aqueous solution, and The solid phase powder (liquid-solid ratio is 0.5mL / g) was stirred and mixed for 2 minutes, and the injectable time was about 3.5 minutes.
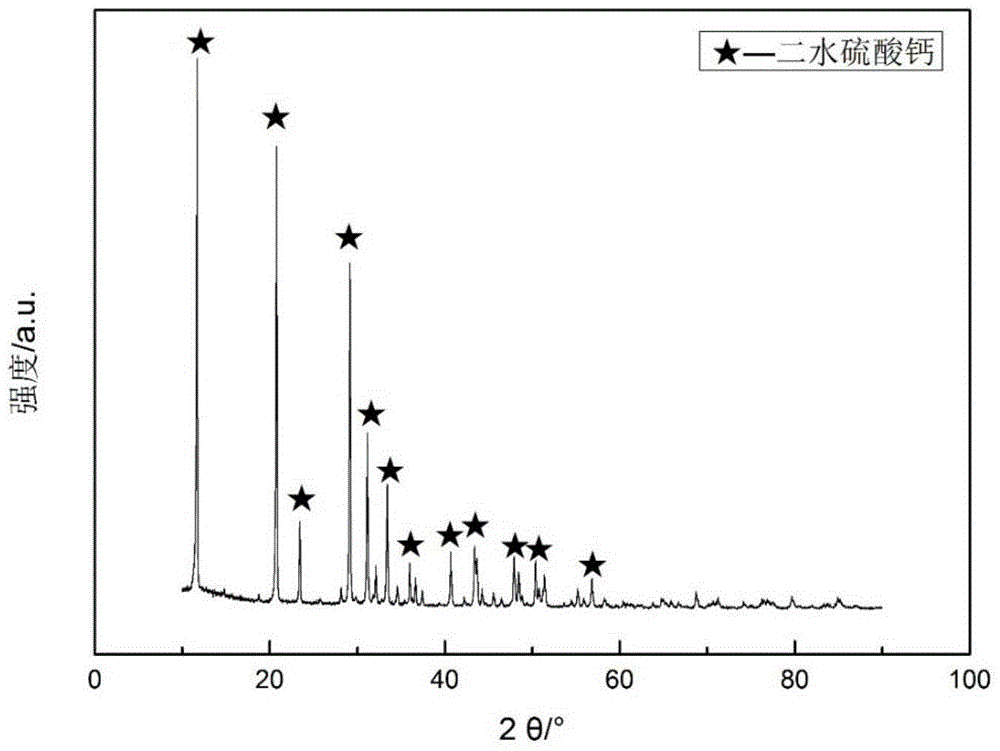
[0029] The cured bone cement was placed in SBF solution for 12 hours, dried and ground for XRD characterization, as shown in the attached figure 1 As shown, the main component of the cured bone cement is calcium sulfate dihydrate, indicating that calcium sulfate hemihydrate quickly turns into calcium sulfate dihydrate during the curing process.
Embodiment 2
[0031] Preparation of bone cement composite powder: at room temperature, weigh 3.992g (99.8%) calcium sulfate hemihydrate and 0.008g (0.2%) polyethylene glycol diacrylate, mix uniformly; Mix 2 mL of thiolated hyaluronic acid aqueous solution with solid phase powder (liquid-solid ratio: 0.5 mL / g) for 2 minutes, and the injection time is about 4 minutes.
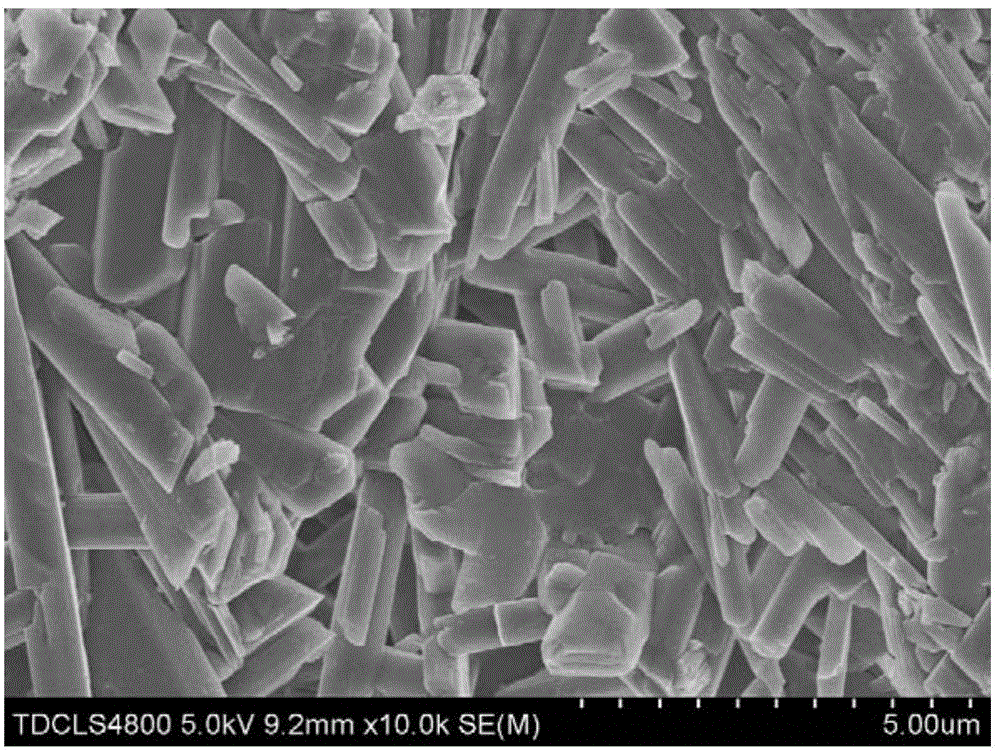
[0032] Carry out phase analysis to cured product according to embodiment 1, and carry out scanning electron microscope observation to the sample after curing, as attached figure 2 As shown, the cured bone cement is distributed in columnar shape.
Embodiment 3
[0034] Preparation of bone cement composite powder: at room temperature, weigh 3.984g (99.6%) calcium sulfate hemihydrate and 0.016g (0.4%) polyethylene glycol diacrylate, mix well; Mix 2 mL of thiolated hyaluronic acid aqueous solution with solid phase powder (liquid-solid ratio: 0.5 mL / g) for 2 minutes, and the injection time is about 4 minutes.
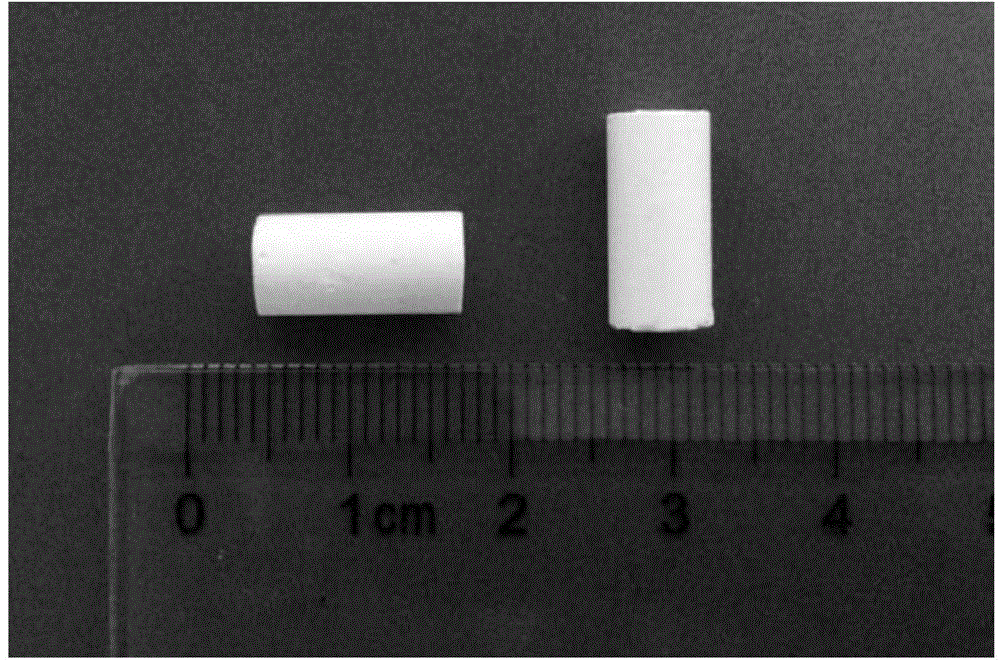
[0035] Carry out phase analysis and scanning electron microscope observation to cured product according to embodiment 2, and carry out compressive strength test to the bone cement sample degraded 14 days, compressive strength test sample is cylindrical (Φ 6mm * 12mm, as attached image 3 shown), the average compressive strength is 5.90Mpa (as attached Figure 4 shown). After curing, soak the bone cement in SBF solution, which has good collapse resistance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com