Method for catalytic reduction of graphene oxide
A technology of graphene and stone oxide, applied in the field of catalytic reduction of graphene oxide, which can solve the problems of poor compatibility, limited use, and influence on the physical and chemical properties of graphene, and achieve the effect of mild reaction conditions and reduced defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
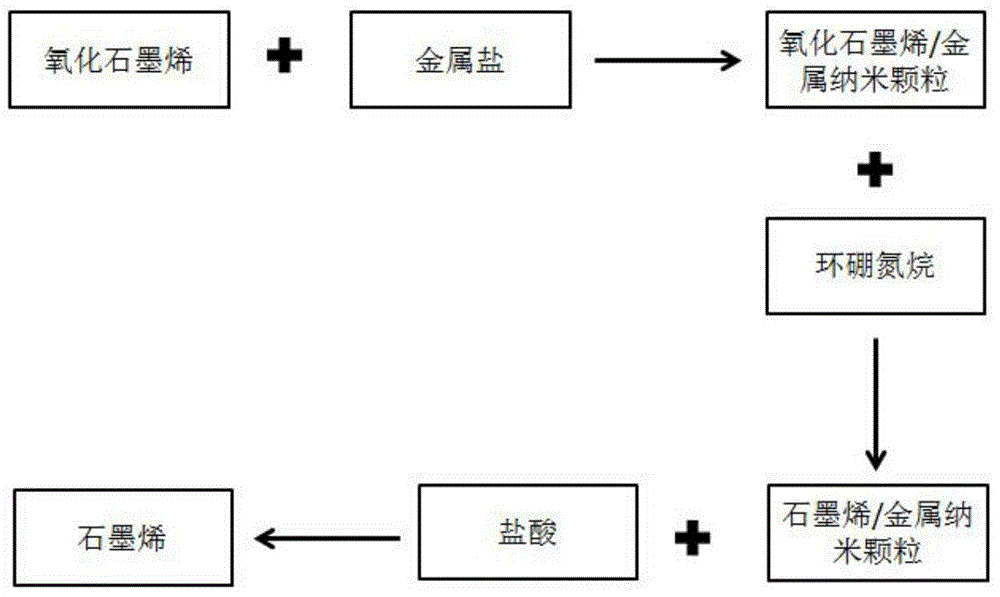
[0023] (1) Preparation of graphene oxide aqueous solution:
[0024] Dissolve 1 mg of graphene oxide into 20 mL of water, disperse and dissolve with 50W ultrasonic to obtain 0.05 mg / mL graphene oxide aqueous solution;
[0025] (2) Add CoCl 2 Solution:
[0026] 2mL of 10 -2 mol / L CoCl 2 The solution is added into the graphene oxide aqueous solution to form a flocculent suspension.
[0027] (3) Add NH 3 BH 3 :
[0028] Add 1 mL of 50 mg / mL NH 3 BH 3 , a black precipitate was obtained, and the size of the metal particles was 2-5nm.
[0029] (4) washing and purifying graphene:
[0030] The black precipitate in step 3 was centrifuged, washed three times with 1 mL of 10% hydrochloric acid, and three times with deionized water, and freeze-dried to obtain reduced graphene oxide powder with a reduction efficiency of 42%.
Embodiment 2
[0032] (1) Preparation of graphene oxide aqueous solution:
[0033] Dissolve 1mg of graphene oxide into 1mL of water, disperse and dissolve with 50W ultrasonic to obtain 1mg / mL graphene oxide aqueous solution;
[0034] (2) Add NiCl 4 Solution:
[0035] 10mL of 10 -4 mol / L NiCl 4 The solution is added into the graphene oxide aqueous solution to form a flocculent suspension.
[0036] (3) Add NH 3 BH 3 :
[0037] Add 2 mL of 25 mg / mL NH 3 BH 3 , a black precipitate was obtained, and the size of the metal particles was 2-5nm.
[0038] (4) washing and purifying graphene:
[0039] The black precipitate in step 3 was centrifuged, washed 3 times with 2 mL of 5% hydrochloric acid, and then 3 times with deionized water, and freeze-dried to obtain reduced graphene oxide powder with a reduction efficiency of 45%.
Embodiment 3
[0041] (1) Preparation of graphene oxide aqueous solution:
[0042] Dissolve 1mg of graphene oxide into 5mL of water, disperse and dissolve with 50W ultrasonic to obtain 0.2mg / mL graphene oxide aqueous solution;
[0043] (2) Add Co(NO 3 ) 2 Solution:
[0044] 5mL of 10 -2 mol / L Co(NO 3 ) 2 The solution is added into the graphene oxide aqueous solution to form a flocculent suspension.
[0045] (3) Add NH 3 BH 3 :
[0046] Add 5 mL of 10 mg / mL NH 3 BH 3 , a black precipitate was obtained, and the size of the metal particles was 2-5nm.
[0047] (4) washing and purifying graphene:
[0048] The black precipitate in step 3 was centrifuged, washed 3 times with 5 mL of 2% hydrochloric acid, and then 3 times with deionized water, and freeze-dried to obtain reduced graphene oxide powder with a reduction efficiency of 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com