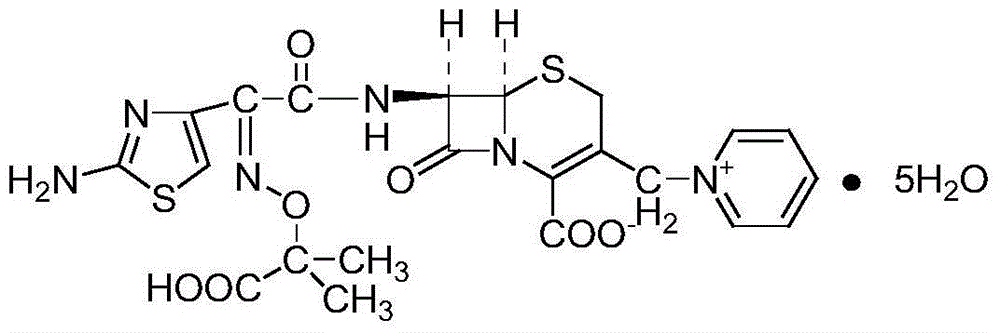
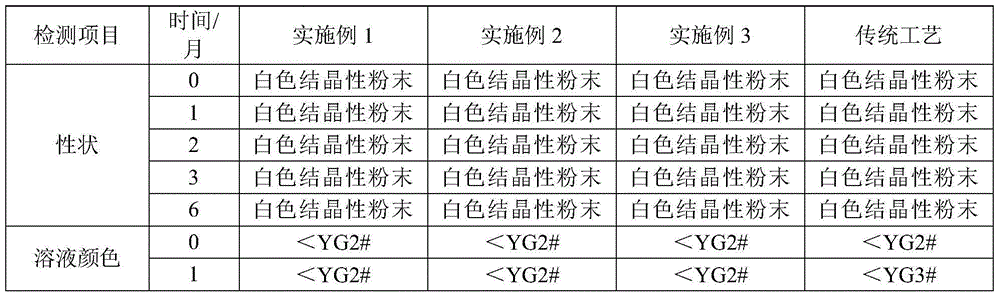
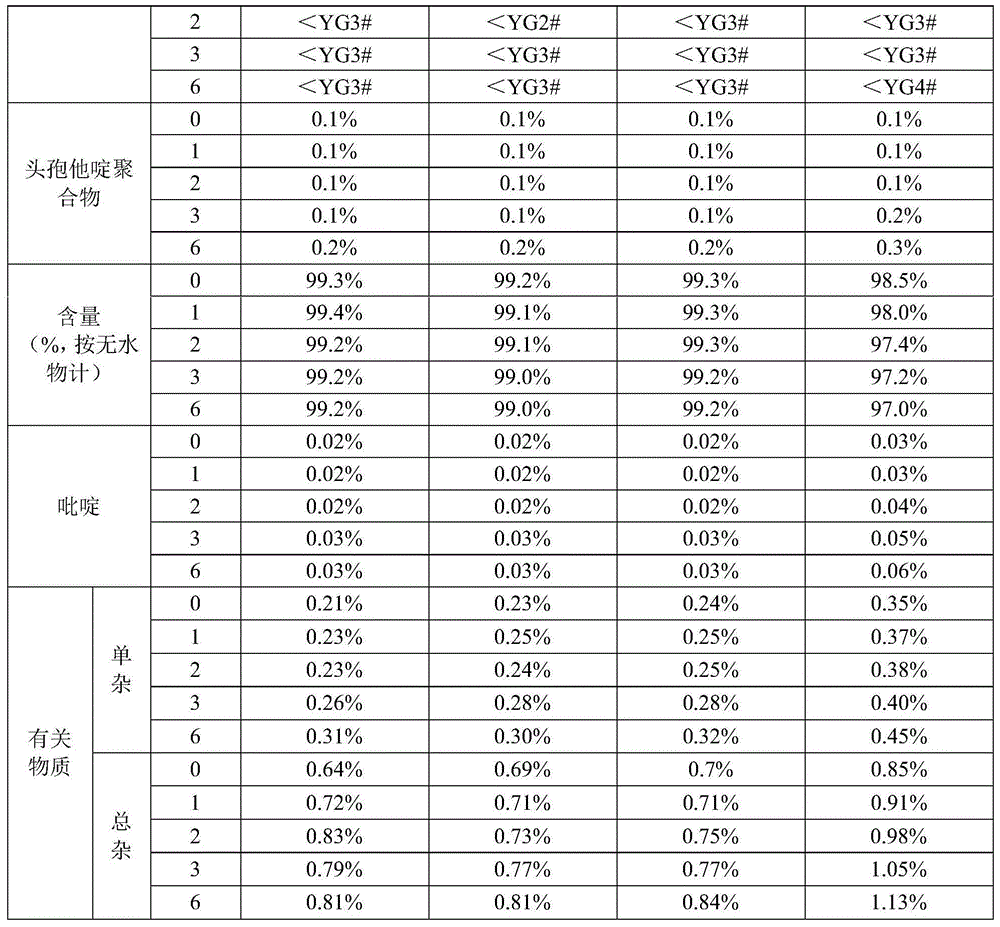
Ceflazidime powder preparation for injection
A technology of ceftazidime and ceftazidime dihydrochloride, which is applied in the field of ceftazidime powder for injection, can solve the problems of many impurities, low purity of active ingredients, obvious side effects, etc., and achieve less residual solvent, less impurities and high stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) At 5°C to 10°C, weigh 150g of ceftazidime dihydrochloride raw material, add 550ml of distilled water, stir to dissolve, cool down to 0°C to 5°C, slowly add 20% sodium hydroxide solution dropwise to adjust the pH to 5.0 ±0.2, add 11g of activated carbon, filter after 30 minutes of decolorization, wash the filter residue and filter bottle with water twice, and the filtrate enters the crystallization bottle.
[0028] (2) At 10°C to 20°C, with a stirring speed of 300 revolutions / min, add hydrochloric acid at the flow acceleration rate shown in the table below:
[0029] time (min)
Drop rate(ml / min)
[0030] 0-10
0.32
10-40
0
40-60
0.6
60-80
1.0
80-110
1.6
110-140
2.1
140-180
0
[0031] (3) suction filtration, wash the filter cake 2 times with 20ml water and acetone respectively, put the filter cake in a vacuum drying oven, the temperature of the vacuum drying oven i...
Embodiment 2
[0034] (1) At 5°C-10°C, weigh 150g of ceftazidime dihydrochloride raw material, add 550ml of distilled water, stir to dissolve, cool down to 0°C-5°C, slowly add ammonia solution dropwise to adjust the pH to 5.0±0.2, add activated carbon 11g, decolorized for 30 minutes and then filtered, washed the filter residue and the filter bottle twice with water, and the filtrate was put into the crystallization bottle.
[0035] (2) At 10°C to 20°C, with a stirring speed of 280 rpm, add 15% phosphoric acid at the flow acceleration rate in the following table:
[0036] time (min)
Drop rate(ml / min)
0-10
0.31
10-40
0
40-60
0.61
60-80
1.0
80-110
1.61
110-140
2.1
140-180
0
[0037] (3) suction filtration, wash the filter cake 2 times with 20ml water and acetone respectively, put the filter cake in a vacuum drying oven, the temperature of the vacuum drying oven is at 40°C, vacuum dry and weigh to ob...
Embodiment 3
[0040] (1) At 5°C-10°C, weigh 150g of ceftazime dihydrochloride raw material, add 550ml of distilled water, stir to dissolve, cool down to 0°C-5°C, slowly add 15% sodium bicarbonate solution dropwise to adjust the pH to 5.0± 0.2, add 11g of activated carbon, filter after 30 minutes of decolorization, wash the filter residue and filter bottle with water twice, and the filtrate enters the crystallization bottle.
[0041] (2) At 10°C to 20°C, with a stirring speed of 280 revolutions / min, add 10% sulfuric acid at the flow acceleration rate in the following table:
[0042] time (min)
Drop rate(ml / min)
0-10
0.33
10-40
0
40-60
0.62
60-80
1.01
80-110
1.6
110-140
2.11
140-180
0
[0043] (3) suction filtration, wash the filter cake 2 times with 20ml water and acetone respectively, put the filter cake in a vacuum drying oven, the temperature of the vacuum drying oven is at 40°C, vacuum dry an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com