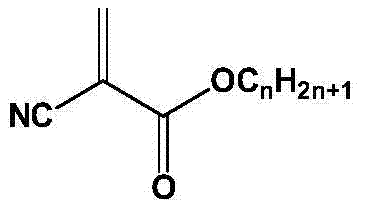
Adhesive and preparation method thereof
An adhesive and equipment technology, which is applied in the field of α-cyanoacrylate-based adhesives and their preparation, can solve the problems of reduced bonding strength, limited viscosity range, complicated processes, etc. Bonding time, the effect of a single product component
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Add 1mL of ethyl α-cyanoacrylate (the purity is 99.9% as determined by gas chromatography normalization method) to each of ten 2mL ampoules, replace the air inside with high-purity argon, and seal it.
[0051] The above 10 ampoule bottles were labeled as 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 in sequence, and bottle No. 1 was used as a blank control sample without ultraviolet crosslinking. Put bottles 2, 3, 4, 5, 6, 7, 8, 9, and 10 into the heating table in turn, set the temperature at 60°C, adjust the height of the UV lamp shelf to 30cm from the sample, and irradiate with a 1000W UV lamp. According to, the time is respectively 10min, 15min, 20min, 25min, 30min, 35min, 40min, 45min, 50min, finally by the rotational viscometer (model is BROOKFIELD VISCOMETER, DV-Ⅱ+Pro, the viscosity value in each embodiment below all uses Measured by this instrument) to test its viscosity. Viscosity test method is: under anhydrous and oxygen-free conditions, take 0.5mL sample and test it in ...
Embodiment 2
[0055] Add 1mL of α-butyl cyanoacrylate (the purity is 99.9% as determined by gas chromatography normalization method) to each of ten 2mL ampoules, replace the air inside with high-purity argon, and seal it.
[0056] The above 10 ampoule bottles were labeled as 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 in sequence, and bottle No. 1 was used as a blank control sample without ultraviolet crosslinking. Put bottles 2, 3, 4, 5, 6, 7, 8, 9, and 10 into the heating table in turn, set the temperature at 60°C, adjust the height of the UV lamp shelf to 30cm from the sample, and irradiate with a 2000W UV lamp. According to the time, the time is 10min, 15min, 20min, 25min, 30min, 35min, 40min, 45min, 50min, and finally the viscosity is tested by a rotational viscometer. The viscosity values measured by the main body of the adhesive are shown in Table 2 below:
[0057] Numbering 1 2 3 4 5 6 7 8 9 10 Viscosity / cps 2.3 4.2 10.6 23.5 38.6 112.6 152.3 / / /
Embodiment 3
[0059] Add 1mL of α-hexyl cyanoacrylate (the purity is 99.9% as determined by gas chromatography normalization method) to each of ten 2mL ampoules, replace the air inside with high-purity argon, and seal it.
[0060] The above 10 ampoule bottles were labeled as 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 in sequence, and bottle No. 1 was used as a blank control sample without ultraviolet crosslinking. Put bottles 2, 3, 4, 5, 6, 7, 8, 9, and 10 into the heating table in turn, set the temperature at 60°C, adjust the height of the UV lamp shelf to 30cm from the sample, and irradiate with a 2000W UV lamp. According to the time, the time is 10min, 15min, 20min, 25min, 30min, 35min, 40min, 45min, 50min, and finally the viscosity is tested by a rotational viscometer. The viscosity value of the main body of the adhesive is shown in the following table 3:
[0061] Numbering 1 2 3 4 5 6 7 8 9 10 Viscosity / cps 2.2 3.9 8.9 22.9 33.5 67.8 111 136.7 / /
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com