Clean production process of 2-thiopheneacetic acid
A technology of thiopheneacetic acid and clean production, applied in the direction of organic chemistry, etc., can solve the problems of unstable intermediate chloromethylthiophene, no application of 2-thiopheneacetic acid, and explosion hazard in reaction treatment, etc., and achieve high product yield and conversion High efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
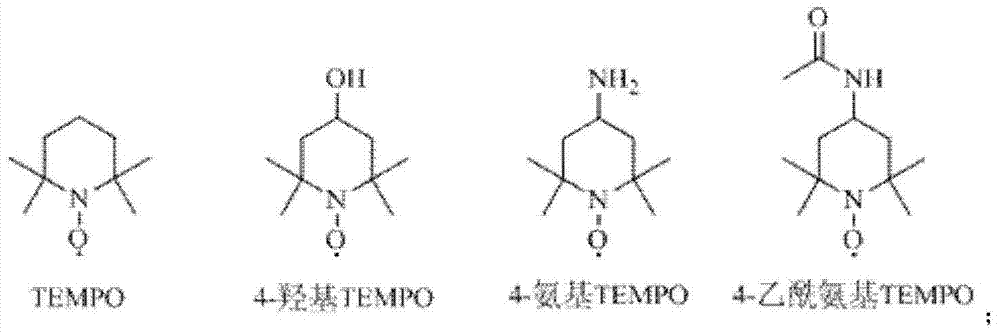
[0036] 10g of 2-thiophene ethanol, 0.4g of TEMPO, 200mL of acetonitrile, 100mL of phosphate buffer (0.6mol.L -1 ), stirred evenly, and the reactor was heated up to 35°C;
[0037] Then slowly add NaClO aqueous solution (16wt%) 80g dropwise to the reactor, after 2h dropwise, after continuing to react for 4h, add 2mol.L -1 Sodium sulfite solution, until NaClO is neutralized completely (starch potassium iodide test paper detects) in the reactor;
[0038] Add 2mol.L -1Adjust the solution to weak alkaline with NaOH, use ethyl acetate to extract the catalyst and unreacted raw materials, and then use 4mol.L -1 HCl adjusts the pH of the solution to 1, and extracts with ethyl acetate to obtain the crude product of 2-thiopheneacetic acid;
[0039] The crude product was recrystallized with petroleum ether to obtain pure 2-thiopheneacetic acid. The purity of the product detected by liquid phase was 99%, the conversion rate of 2-thiophene ethanol was 100%, the reaction selectivity was 95...
Embodiment 2
[0041] 10g of 2-thiophenethanol, 0.2g of 4-hydroxy TEMPO, 100mL of acetonitrile, 100mL of phosphate buffer (0.6mol.L -1 ), stirred evenly, and the reactor was cooled to 0°C;
[0042] Then NaClO was slowly added dropwise to the reactor 2 Aqueous solution (20wt%) 70.6g, 1.5h was added dropwise, after continuing to react for 3h, add 2mol.L -1 Sodium sulfite solution until NaClO in the reactor 2 Completely neutralized (detected by starch potassium iodide test paper);
[0043] Add 2mol.L -1 Adjust the solution to weak alkaline with NaOH, use ethyl acetate to extract the catalyst and unreacted raw materials, and then use 4mol.L -1 HCl adjusted the pH of the solution to 1.5, and extracted with ethyl acetate to obtain crude 2-thiopheneacetic acid;
[0044] The crude product was recrystallized from petroleum ether to obtain pure 2-thiopheneacetic acid, the conversion rate of 2-thiophenethanol was 92%, the reaction selectivity was 90%, and the yield was 73%.
Embodiment 3
[0046] 10g of 2-thiophene ethanol, 0.6g of 4-amino TEMPO, 300mL of acetonitrile, 100mL of phosphate buffer (0.6mol.L -1 ), stirred evenly, and the reactor was heated up to 50°C;
[0047] Then in the reactor, 138g of potassium hydrogen persulfate aqueous solution (50wt%) was slowly added dropwise, and the dropwise addition was completed in 2.5h. After continuing the reaction for 5h, 2mol.L -1 Sodium sulfite solution until potassium persulfate in the reactor is completely neutralized (detected by starch potassium iodide test paper);
[0048] Add 2mol.L -1 Adjust the solution to weak alkaline with NaOH, use ethyl acetate to extract the catalyst and unreacted raw materials, and then use 4mol.L -1 HCl adjusted the pH of the solution to 1.7, and extracted with ethyl acetate to obtain the crude product of 2-thiopheneacetic acid;
[0049] The crude product was recrystallized from petroleum ether to obtain pure 2-thiopheneacetic acid, the conversion rate of 2-thiophenethanol was 90%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com