Novel efficient and reversible ion type ammonia gas absorbent
An absorbent and ionic technology, applied in the field of Lewis acid ionic liquid, to achieve complete desorption, stable absorption performance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
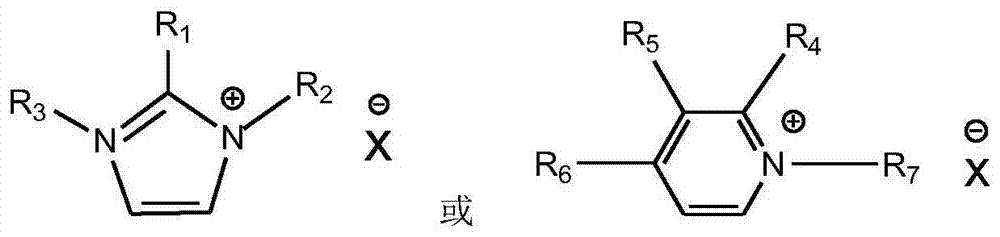
[0015] Add 1mol of N-methylimidazole into a 500mL three-necked round-bottomed flask with a condenser, stir it magnetically to raise the reaction temperature to 65°C, then slowly add 1.05mol of n-chlorobutane dropwise, and the dropwise addition is complete Afterwards, the temperature was raised to 70°C, and the reaction was heated under reflux for 48h. Take it out and extract it with ethyl acetate three times, and the ionic liquid phase is removed by a rotary evaporator to remove the residual organic solvent to obtain the [Bmim][Cl] intermediate, which is a white waxy solid at room temperature. [Bmim][Cl] (21.53g, 0.12mol), anhydrous cobalt chloride (8.00g, 0.062mol) and potassium thiocyanate (23.95g, 0.25mol) were dissolved in 50ml of acetone and stirred at room temperature for 24h. After the reaction, acetone was filtered and rotary evaporated, added dichloromethane and placed in the refrigerator for 2 hours, filtered and rotary evaporated again to remove the solvent, and vac...
Embodiment 2
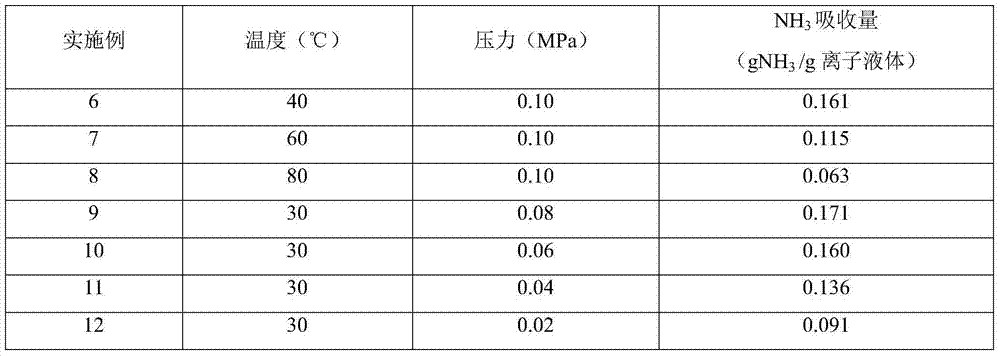
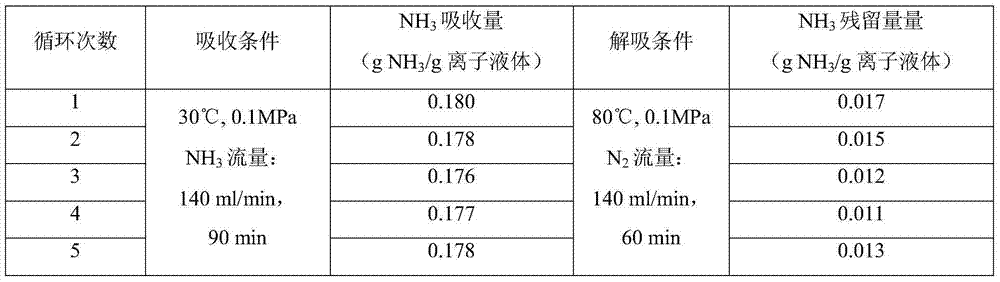
[0018] 1) In a self-made absorption bottle with an internal diameter of 3.00cm, add 5.00g of the ionic liquid [Bmim] synthesized in 1) in Example 1 2 [Co(NCS) 4 ], and then into pure NH 3 , the gas flow rate is 140ml / min, the temperature is 30°C, and the pressure is 0.1MPa. Take the weight of the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. Calculate the ammonia absorption capacity in the ionic liquid to be 0.180g NH 3 / g ionic liquid (6.028mol NH 3 / mol ionic liquid).
[0019] 2) In a self-made absorption bottle with an inner diameter of 3.00cm, add 5.00g of ionic liquid N-butylimidazole thiocyanate [Bmim][SCN], and then pass pure NH 3 , the gas flow rate is 140ml / min, the temperature is 30°C, and the pressure is 0.1MPa. Take the weight of the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. The calculated...
Embodiment 3
[0021] 1) Add 5.00g of ionic liquid [Emim] into a self-made absorption bottle with an inner diameter of 3.00cm 2[Co(NCS) 4 ], and then into pure NH 3 , the gas flow rate is 140ml / min, the temperature is 30°C, and the pressure is 0.1MPa. Take the weight of the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. The calculated ammonia absorption capacity in the ionic liquid is 0.198gNH 3 / g ionic liquid (5.999molNH 3 / mol ionic liquid).
[0022] 2) In a self-made absorption bottle with an inner diameter of 3.00cm, add 5.00g of ionic liquid N-ethylimidazole thiocyanate [Emim][SCN], and then pass pure NH 3 , the gas flow rate is 140ml / min, the temperature is 30°C, and the pressure is 0.1MPa. Take the weight of the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. The calculated ammonia absorption capacity in the ionic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com