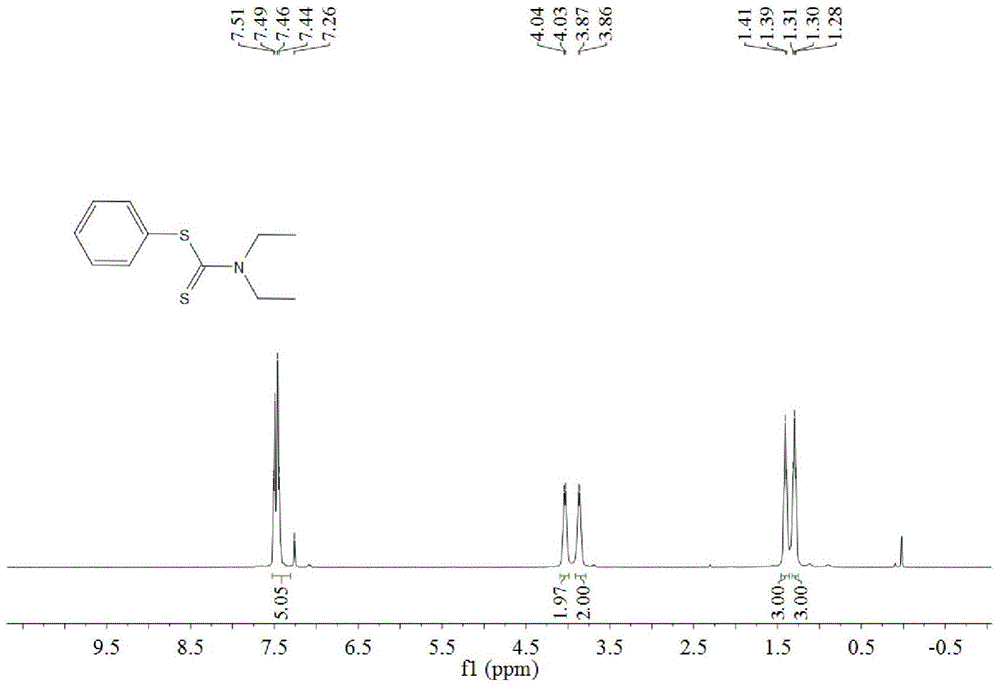
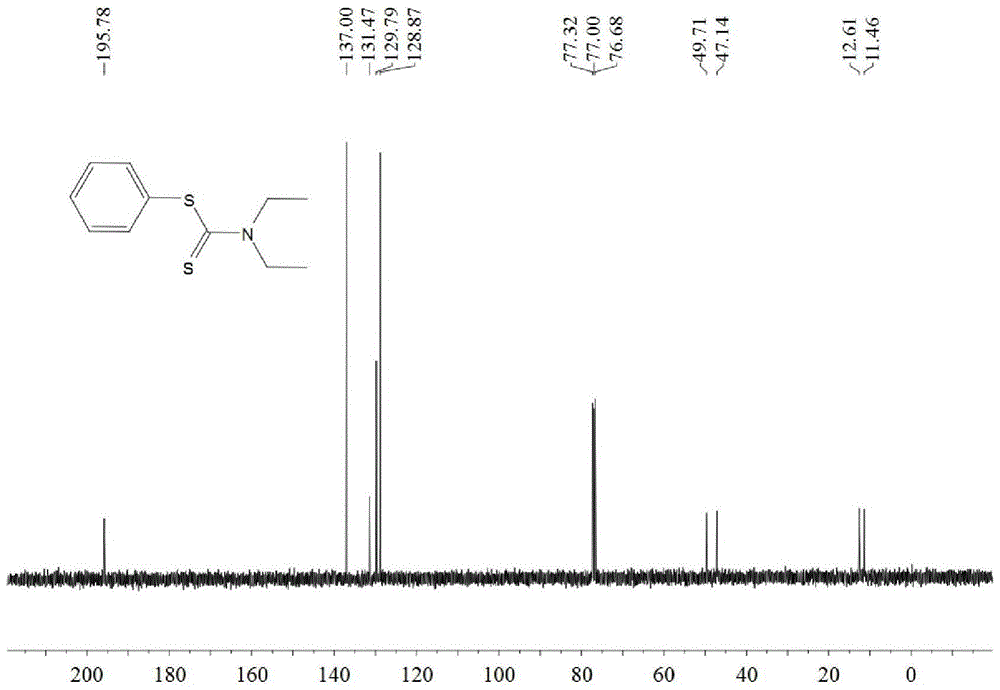
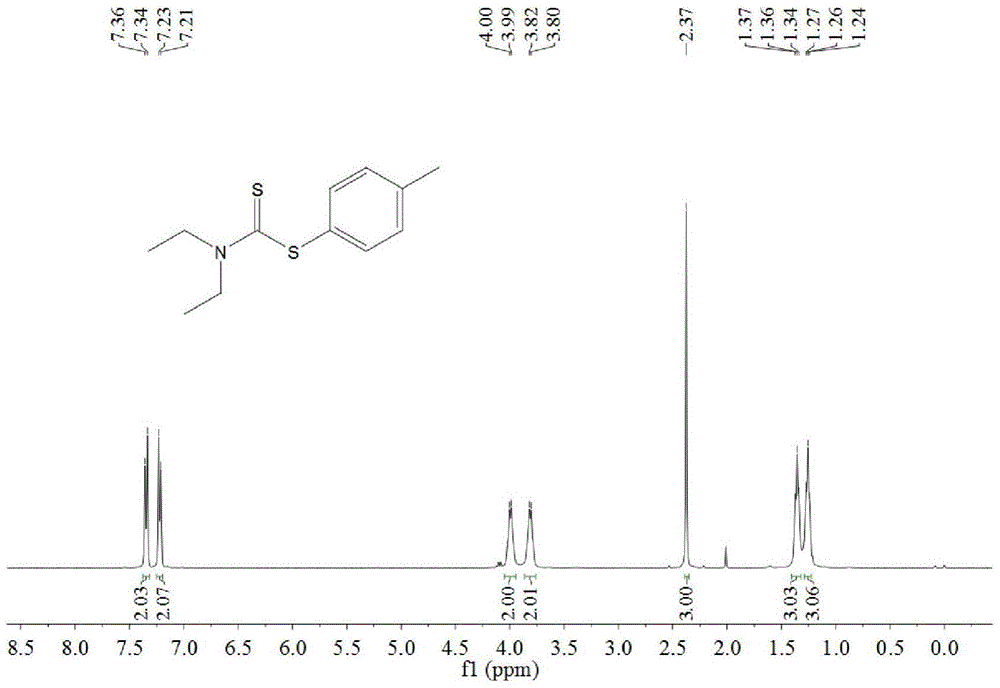
A kind of synthetic method of dithiocarbamate
A technology of dithiocarbamate and a synthesis method, which is applied in directions such as organic chemistry, can solve the problems of low atom utilization rate, harsh reaction conditions, complicated operation steps, etc., and achieves large industrial production value, and the method is simple and easy to implement. The effect of cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 0.5 mmoles of phenylboronic acid, 1 mmoles of carbon disulfide, 2.5 mmoles of diethylamine, 1.5 mmoles of potassium carbonate, 0.5 mmoles of copper acetate, and 3 ml of acetonitrile into the reaction tube, stir and react at 60°C for 10 hours, then stop heating And stir, cool to room temperature. The reaction liquid was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. The rate is 88%.
Embodiment 2
[0043] Add 0.5 mmol of phenylboronic acid, 1 mmol of carbon disulfide, 2.5 mmol of diethylamine, 1.5 mmol of potassium carbonate, 0.5 mmol of copper acetate, and 3 ml of acetonitrile into the reaction tube, stir and react at 120°C for 10 hours, then stop heating And stir, cool to room temperature. The reaction liquid was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. rate of 74%.
Embodiment 3
[0045] Add 0.5 mmol of phenylboronic acid, 1 mmol of carbon disulfide, 2.5 mmol of diethylamine, 1.5 mmol of potassium carbonate, 0.5 mmol of copper chloride, and 3 ml of acetonitrile into the reaction tube, and stir the reaction at 60°C for 24 hours, then stop Heat and stir, and cool to room temperature. The reaction liquid was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. Rate 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com