Application of artemisinin and derivatives thereof in preparation of ophthalmic vascular disease prevention and treatment medicines, and medicinal composition
A technology of artemisinin and its derivatives, applied in the field of ophthalmic vascular diseases, can solve the problems of peripheral visual field damage, decreased treatment effect, drug tolerance, etc. The effect of reducing complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
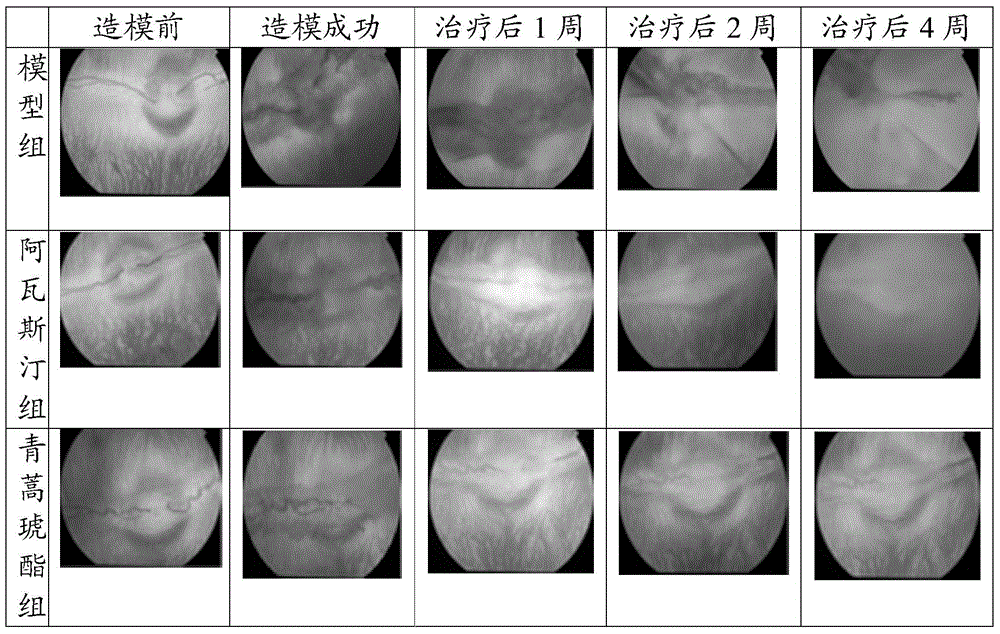
[0028] The application of artesunate in the preparation of drugs for the treatment of retinal choroidal neovascularization specifically includes the following:
[0029] (1) Dosing: mix artesunate and 0.05% sodium bicarbonate solution to make a 0.1-6mg / ml gradient solution;
[0030] (2) Dose: 0.1-0.5ml;
[0031] (3) Operation steps:
[0032] A. Disinfection of the surgical area: Disinfect the eyelids and skin around the eyes with 10% povidone-iodine, put 3 drops of 0.5% povidone-iodine in the conjunctival sac for a few minutes, rinse the conjunctival sac with tobramycin solution, and use antibiotic drops Eye drops to prevent dryness and abrasion of the cornea.
[0033] B. Local anesthesia: Put 3 to 4 drops of Alcaine eye drops into the conjunctival sac for local anesthesia.
[0034] C. Vitreous intravitreal injection: The injection site should be located 3.5-4 mm behind the limbus. The syringe uses a 30-gauge needle to pass through the surface of the sclera obliquely, and th...
Embodiment 2
[0036] (1) In vitro test
[0037] 1. Purpose of the experiment
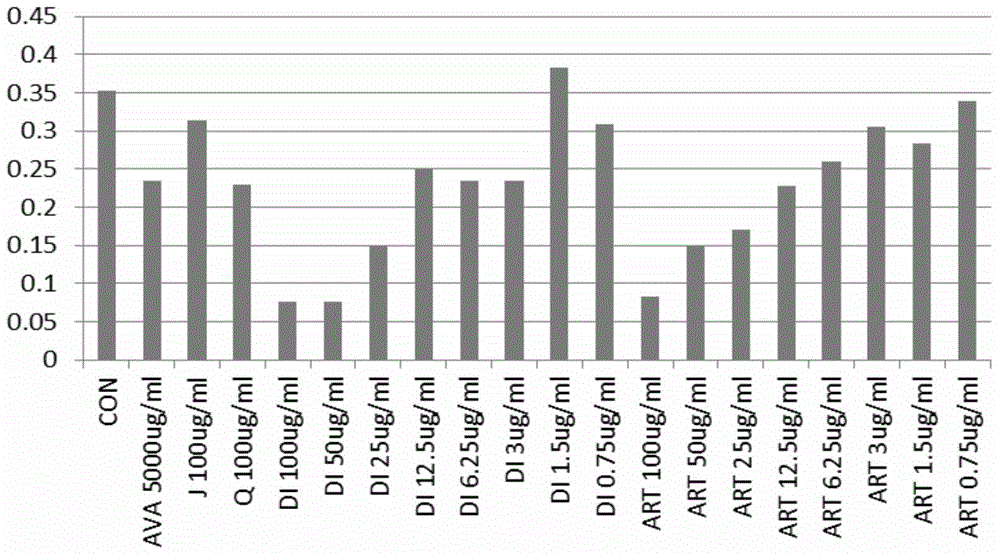
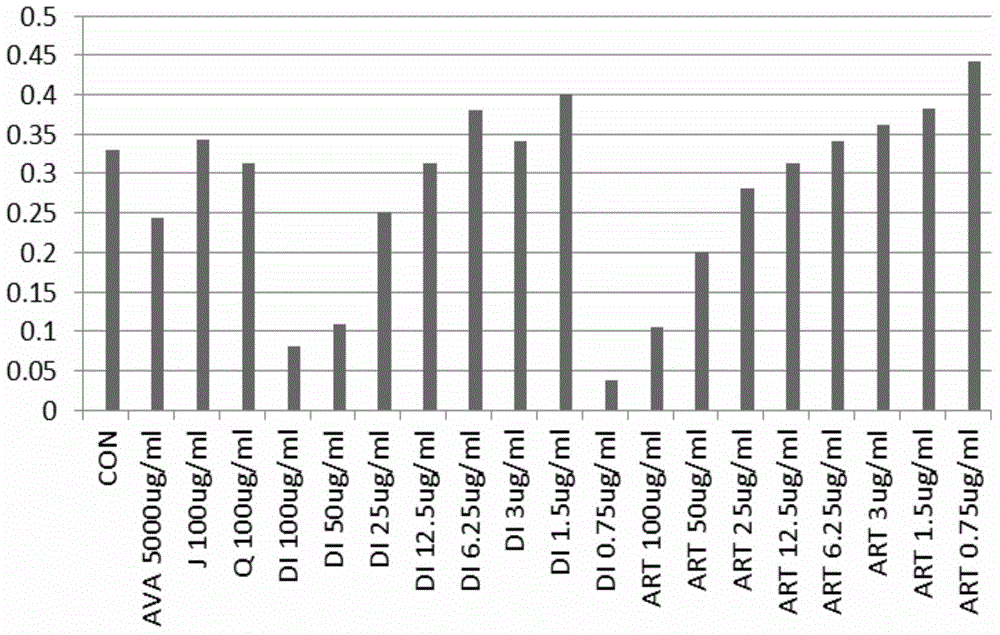
[0038]Through in vitro experiments, the effects of artemisinin (Q) and its derivatives (artesunate (ART), dihydroartemisinin (DI) and artemether (J)) on HUVEC and RF / 6A were analyzed, namely Analyze the inhibition of HUVEC and RF / 6A proliferation and induction of HUVEC and RF / 6A apoptosis by artemisinin and its derivatives, so as to further screen the most effective artemisinin derivative monomers, calculate IC50, and find the appropriate concentration.
[0039] 2. Experimental method
[0040] (1) Human umbilical vein endothelial cells (human umbilical vein endothelial cells, HUVEC) were extracted for primary culture, and the 2nd-4th passage cells were used.
[0041] (2) Artemisinin and its derivative monomers induce HUVEC apoptosis experiment
[0042] Subjects: HUVEC, rhesus monkey retinal choroidal vascular endothelial cells (RF / 6A);
[0043] The experimental method is as follows, apoptosis test (MTT), repe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com