Human Annexin V genetic optimization sequence and preparation method and application thereof
A technology for gene optimization and sequence optimization, applied in the field of bioengineering, can solve problems such as difficult recombination expression, and achieve the effects of reducing production costs, simple equipment and process, and easy large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of optimized method of human Annexin V gene:
[0032] Log in to GeneBank and search for the gene sequence encoding the mature protein of human Annexin V (sequence number: GenBank: AK312644.1). Its nucleotide length is 963bp. Since human Annexin V is a eukaryotic protein, follow-up research needs to be carried out in prokaryotes Therefore, the coding codons of the above-mentioned human Annexin V gene were compared with the preferred codons of E. coli. According to the degeneracy of the codons, on the basis of not changing the sequence of the amino acid composition, the known human Annexin V coding The gene sequence is modified, and some rare codons of E. coli are replaced with preferred codons of E. coli to improve the expression level of the target gene in E. coli, and the transgenic verification is carried out. The finally obtained human Annexin V gene optimized sequence is shown as SEQ ID NO: 1.
[0033] Add the NdeI restriction site to the 5′ end of the human...
Embodiment 2
[0035] 2.1 Obtaining expression strains
[0036] The prokaryotic expression plasmid pET23a-ANXV obtained in Example 1 was transformed into Escherichia coli BL21 (DE3) (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) by the heat shock method, and ampicillin was added to the LB plate medium to 100 mg / L was screened, and a single colony was picked for sequencing identification.
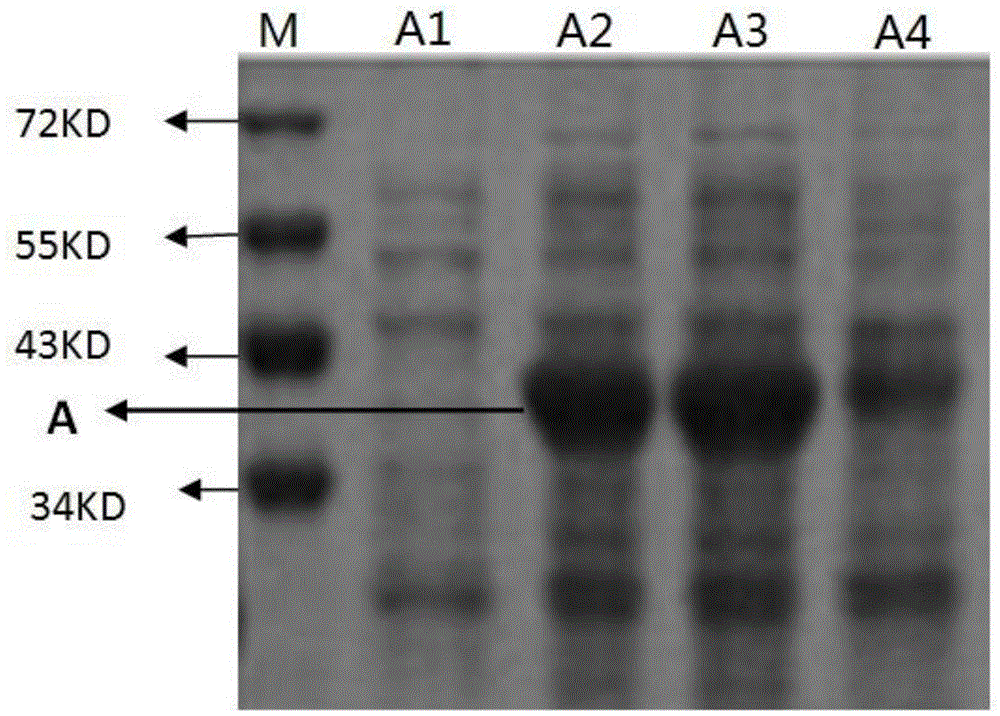
[0037] 2.2 Induced expression of human Annexin V gene optimized sequence in Escherichia coli
[0038] Pick a single colony containing the recombinant positive plasmid, inoculate it into LB liquid medium containing 100mg / L ampicillin, culture overnight at 37°C and 220rpm with shaking, and inoculate the overnight cultured bacterial solution to 100mg / L ampicillin fresh LB liquid medium, 37 ° C, 220rpm shaking culture to the bacterial concentration OD 600 About 0.6, take out 2mL of the bacterial liquid as the uninduced control, immediately add the inducer IPTG to the working concentration of 1...
Embodiment 3
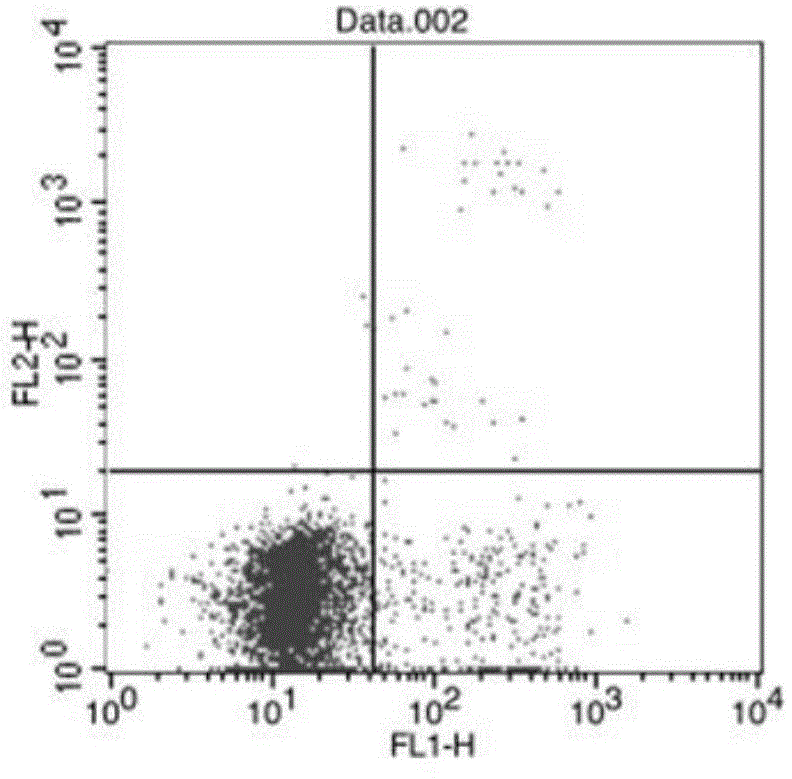
[0043] Human Annexin V FITC labeling and apoptosis detection
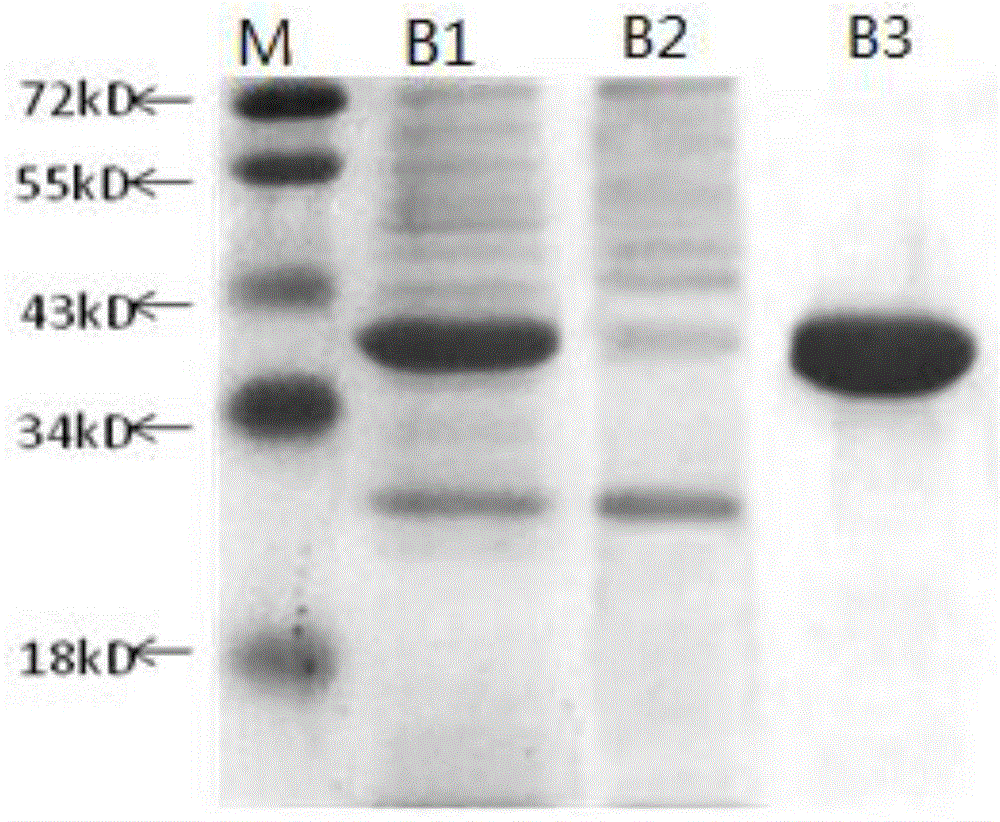
[0044] 3.1 Human Annexin V FITC labeling
[0045] Take 5 mg of human Annexin V recombinant protein, and use a dialysis bag with a cut-off molecular weight of 12-14KDa for overnight dialysis (buffer 0.1M Na 2 CO 3 -NaHCO 3 , pH9.5) dialyzed, during which the buffer was changed 3 times; 4°C, 12000rpm, centrifuged for 15min; the supernatant was taken to measure the concentration. Dissolve FITC in DMSO to a final concentration of 5mg / mL; add FITC to the protein according to the ratio of 9uL FITC per mg of human Annexin V recombinant protein, mix with a vortex mixer, wrap with tinfoil, and rotate in a hybridization oven at room temperature in the dark Reaction for 2 hours; equilibrate Sephadex G25 chromatography column with 5 times column bed volume deionized water; 5 times column bed volume Annexin V-FITC storage buffer (50mM Tris, 150mM NaCl, 0.05%NaN 3 , pH8.0) to equilibrate the Sephadex G25 chromatography colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com