Synthesis method for 2,3,5,6-tetrafluorophenol
A synthesis method and technology of tetrafluorophenol are applied in the field of synthesis of 2,3,5,6-tetrafluorophenol, can solve the problems of unavailability, industrial production limitation, expensive pentafluorobenzene series compounds and the like, and achieve the reaction step The effect of short, good product purity and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
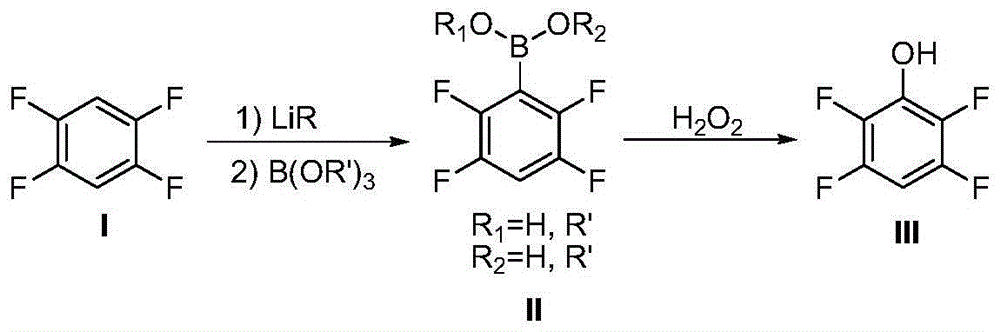
[0030] To a 500ml dry reaction flask, replace the gas with nitrogen, add 22.5g of compound (I) and 90g of anhydrous ether, under the protection of nitrogen, stir and cool to -70~-80°C, slowly add 1.6M n-butyl Lithium-based n-hexane solution of 105 ml, after the dropwise addition was completed, the temperature was controlled at -60 to -70°C and the reaction was stirred for 2 hours. The temperature of the system was lowered to -70~-80°C, and 20.3 g of trimethyl borate was added dropwise. After the dropwise addition, the temperature was controlled at -60~-70°C to continue the reaction for 5 hours. Return the reaction system to room temperature, and slowly dilute it into 100 grams of 10% hydrochloric acid aqueous solution. After the dilution is complete, control the temperature at 20-30°C, add 21.9 grams of 35% hydrogen peroxide solution dropwise, and stir at 20-30°C. 30 hours. Add 60 grams of 10% sodium bisulfite solution to the reaction system, stir at room temperature for 1 ho...
Embodiment 2
[0032] In a 500ml dry reaction flask, replace the gas with nitrogen, add 15.0g of compound (I) and 180g of anhydrous toluene, under the protection of nitrogen, stir and cool to -60~-70°C, slowly add 1.5M n-propyl Lithium-based n-hexane solution of 87 ml, after the dropwise addition was completed, the temperature was controlled at -50 to -60°C and the reaction was stirred for 1 hour. Add 36.8 g of tri-n-butyl borate dropwise to the reaction system. After the dropwise addition, react at -50 to -60°C for 1 hour, then raise the temperature to -20 to -30°C to continue the reaction for 2 hours. Return the reaction system to room temperature, slowly dilute it into 100 g of 10% sulfuric acid aqueous solution, and recover toluene by distillation under reduced pressure after dilution, adjust the pH of the residual system to 4.0 with 10% sodium hydroxide solution, and add 15% hydrogen peroxide dropwise 45.3 g of aqueous solution, after the dropwise addition, the temperature of the system...
Embodiment 3
[0034]In a 500ml dry reaction flask, replace the gas with nitrogen, add 30.0g of compound (I) and 180g of anhydrous 2-tetrahydrofuran, under the protection of nitrogen, stir and cool to -70~-80°C, slowly add 2.5M 84 ml of n-hexane solution of n-butyllithium, after the dropwise addition was completed, the temperature was controlled at -60 to -70°C and the reaction was stirred for 1 hour. 41.4 g of tri-n-propyl borate was added dropwise. After the dropwise addition was completed, the temperature was controlled at -60 to -70°C and the reaction was stirred for 1 hour, and the temperature was raised to -30 to -40°C to continue the reaction for 3 hours. The reaction system was warmed to room temperature, and 100 grams of 12% sulfuric acid aqueous solution was added. After the addition, 32.6 grams of 25% hydrogen peroxide aqueous solution was added dropwise, and the system was heated to 30-40° C. and stirred for 20 hours. The reaction system was cooled down to room temperature, the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com