Method and liquid mass database for detecting residual chemicals in animal-derived food
A technology for detecting animals and animal sources, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the detection and verification rules of various types of residues are not yet perfect, lack of matrix standard data, and residue forms are different.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1. Preparation of standard working solution
[0093] Steroid hormone compounds, nitroimidazole drugs and their metabolites, and Beta-receptor agonist substances: use acetonitrile to prepare their mixed standard stock solutions (0.01g / L), and the substance standard stock solutions can be stable for 12 moon.
[0094] Dyestuffs and other substances are classified into methanol to prepare their mixed standard stock solution (0.01g / L), which can be stable for 3 months.
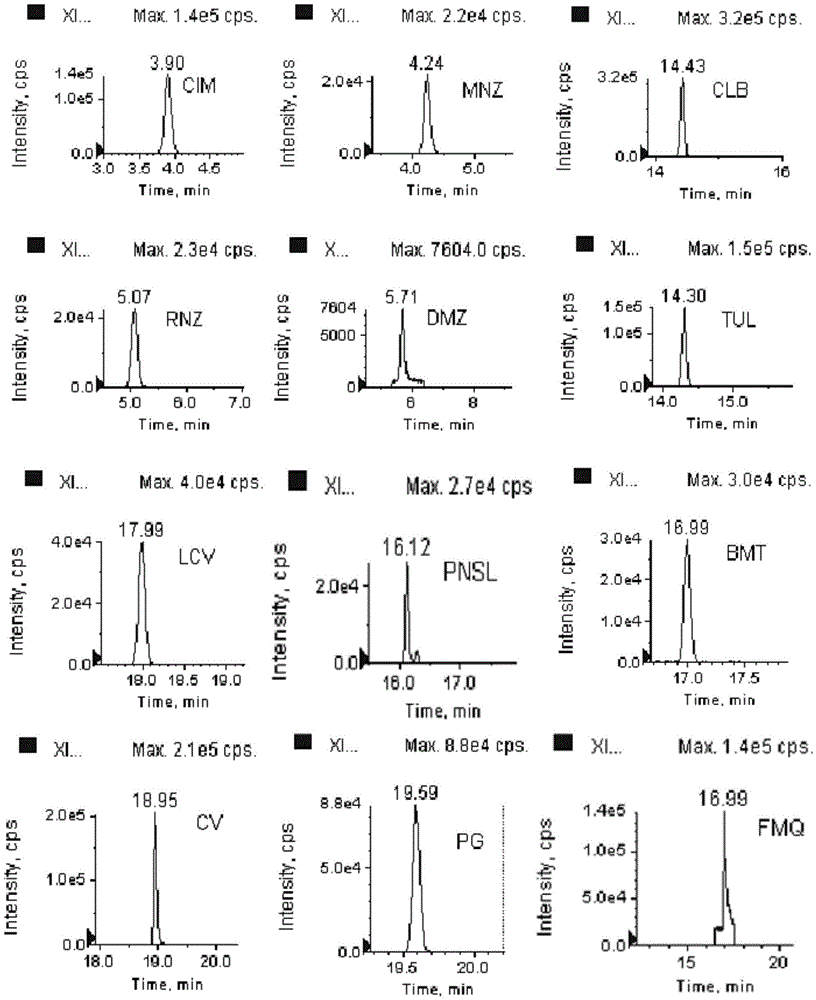
[0095] Mix and standard working solution (0.1mg / L), respectively pipette testosterone, prednisolone, betamethasone, dexamethasone, metronidazole, ronidazole, dimetridazole, clenbuterol, Appropriate amount of buterol, cimaterol, crystal violet and leuco crystal violet standard stock solutions are used, and a mixed standard solution with a concentration of 0.1mg / L is prepared with acetonitrile. The solution is stable at -20°C for 1 month.
Embodiment 2
[0096] Embodiment 2. Analysis pretreatment method
[0097] The 12 target compounds (Group A substances) belong to 4 compound categories, including steroid hormones, nitroimidazoles, β-receptor agonists and dyes, and their implementation limit requirements range from 0.05 μg / kg to 0.8 μg / kg is a group of drugs with the most stringent limit requirements in the world. Therefore, the steps of purification and concentration were considered in the design of the pretreatment process to meet the strict limit requirements. Aiming at the types of compounds involved in Group A substances, the extraction and purification technology of rapid enzymatic hydrolysis (release of bound state residues) + rapid solid phase extraction (SPE) was designed, which can quickly and effectively complete the pretreatment process of 12 ultra-low limit substances . The specific extraction and purification process is as follows:
[0098] (1) Extraction: Weigh 5g of homogeneous sample (accurate to 0.01g) an...
Embodiment 3
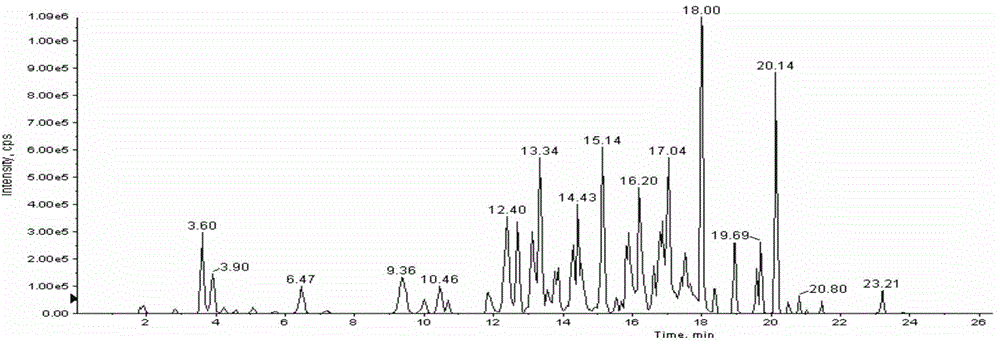
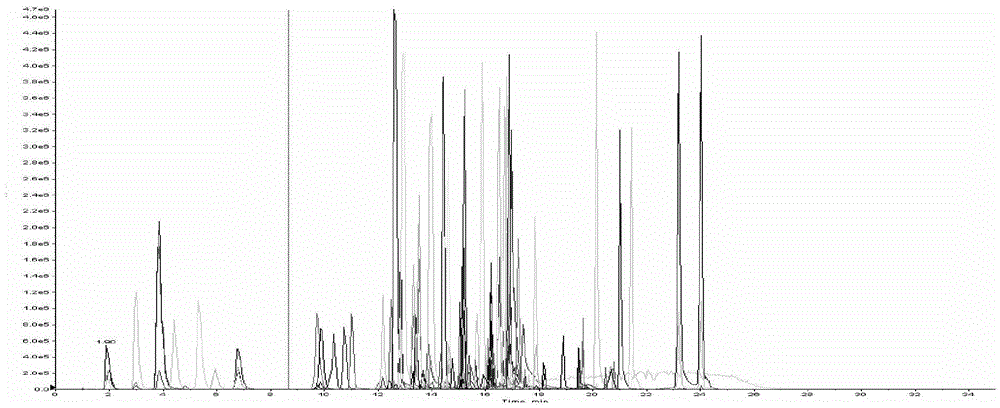
[0103] Embodiment 3. Research on one-time sampling chromatographic analysis system
[0104] 1. Selection of chromatographic column
[0105] Ultrafine particle (particle size <2 μm) chromatographic columns are usually considered for liquid chromatographic separation of multi-target analytes. However, this study uses a conventional HPLC system with a pressure upper limit of 400 Bar, so ultra-high performance liquid chromatography columns cannot be used. In order to achieve the separation purpose of the design, considering the system pressure limitation, the particle size range of the chromatographic column is controlled between 2 μm and 3 μm, and the length of the chromatographic column is 100 mm to 150 mm; In terms of chromatographic column type, a C18 chromatographic column suitable for separating a wide polarity range was selected. Through the above limitations, this study selected one of each type of compound based on polarity (selected with reference to the LogD value), an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com