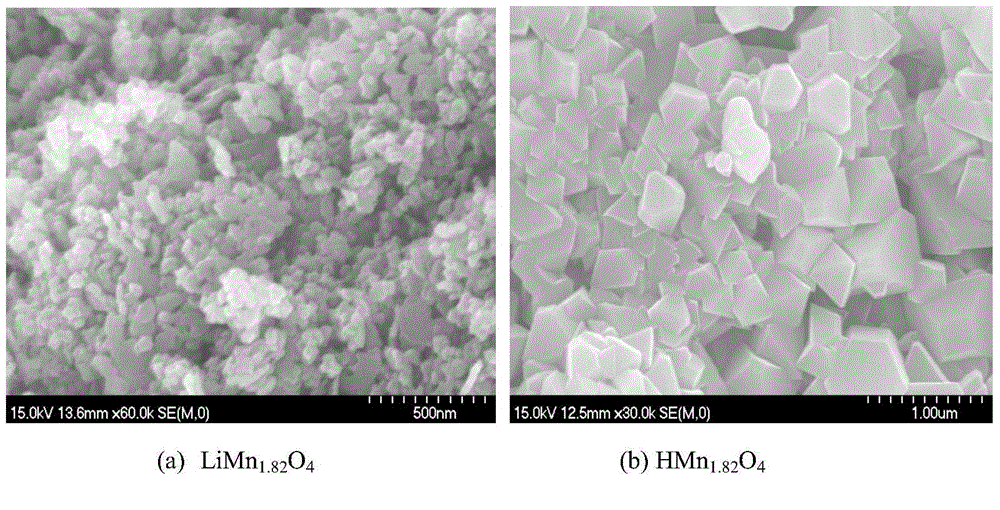
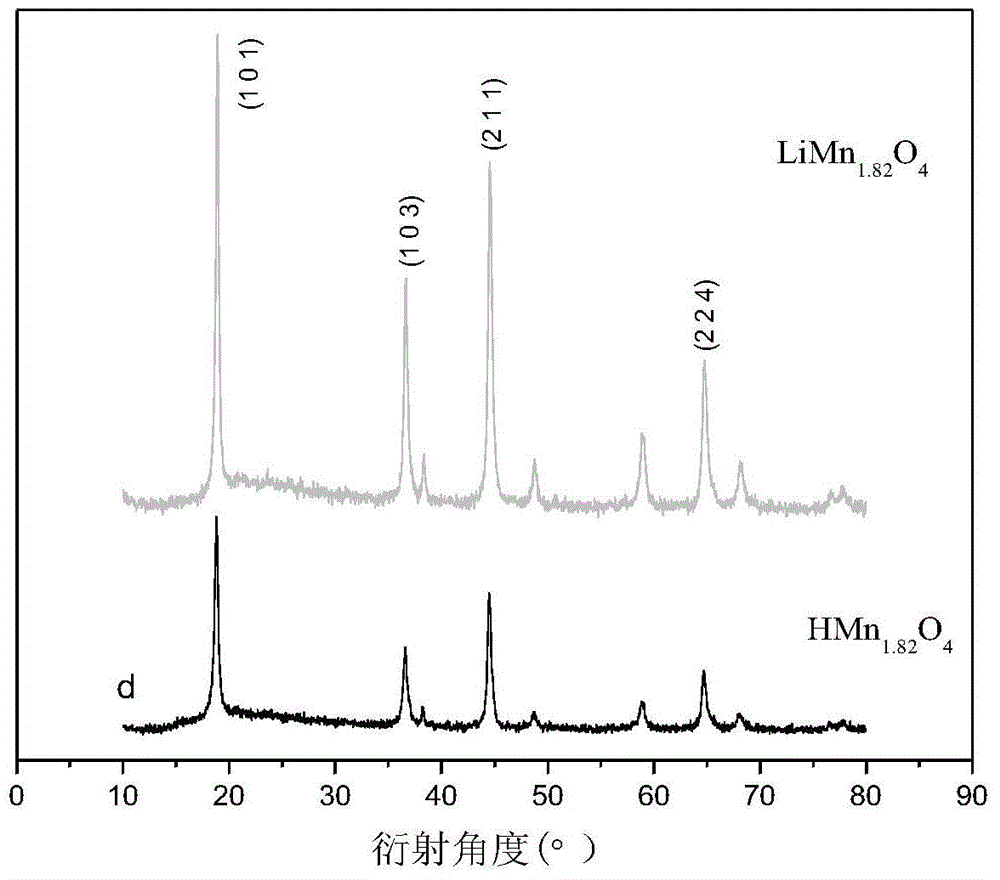
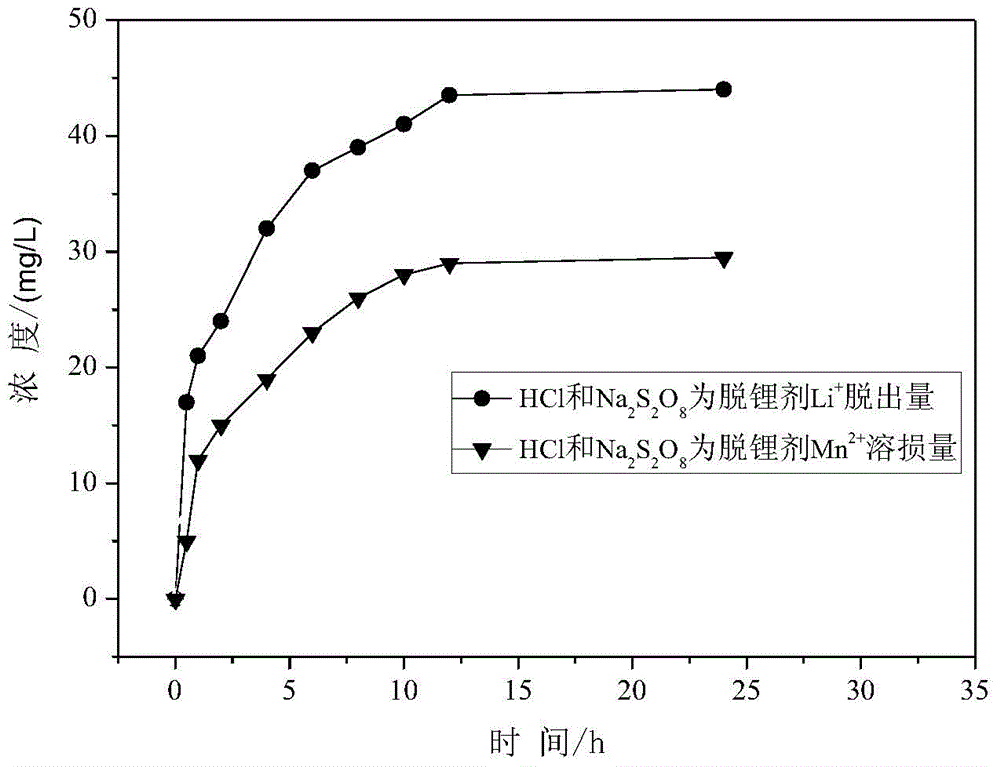
Method for preparing manganese-based lithium-ion sieve adsorbent
A lithium ion and adsorbent technology, which is applied in the field of preparation of manganese-based lithium ion sieve adsorbents, can solve the problems of low recycling rate of ion sieves, low purity of reaction products, unfavorable large-scale production, etc. Control, dissolution loss reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Accurately weigh 8.3920g of analytically pure lithium hydroxide monohydrate, add it to 60mL of ethanol solution with a volume fraction of 3%, and dissolve it, then add 24.5101g of manganese acetate tetrahydrate to it, and react at room temperature for 2 hours under the action of magnetic stirring to obtain a brick Red precipitated product, then drop 6.0mL of 30% H 2 o 2 solution, after reacting for 20 minutes, add 2.0980 g of lithium hydroxide monohydrate to it, stir and react for 12 hours, then transfer the cloudy solution to a polytetrafluoroethylene-lined reactor, and react at a constant temperature of 120°C for 12 hours. The resulting precipitate was filtered, washed, and dried to obtain a reddish-brown intermediate product, which was ground to uniform and fine particles, then placed in a muffle furnace at a rate of 10°C / min to 300°C, kept at a constant temperature for 5 hours, and then cooled naturally to room temperature, the spinel-type lithium-ion sieve precurs...
Embodiment 2
[0042] Accurately weigh 8.3920 g of analytically pure lithium hydroxide monohydrate, add it to 70 mL of ethanol solution with a volume fraction of 5% and dissolve it, then add 24.5101 g of manganese acetate tetrahydrate therein, and react at room temperature for 3 h under the action of a magnetic stirrer to obtain Brick red precipitation product, then drop 6.0mL mass fraction of 30% H into it at a speed of 1.0mL / min 2 o 2 solution, after reacting for 20 minutes, 3.1472 g of lithium hydroxide monohydrate was added thereto, stirred and reacted for 12 hours, then the cloudy solution was transferred to a polytetrafluoroethylene-lined reactor, and reacted at a constant temperature of 140°C for 12 hours. The resulting precipitate was filtered, washed, and dried to obtain a reddish-brown intermediate product, which was ground to uniform and fine particles, then placed in a muffle furnace at a heating rate of 10°C / min to 400°C, kept at a constant temperature for 5 hours, and then cool...
Embodiment 3
[0044] Accurately weigh 8.3920 g of analytically pure lithium hydroxide monohydrate and add it to 80 mL of ethanol solution with a volume fraction of 8% to dissolve it, then add 24.5101 g of manganese acetate tetrahydrate to it, and react at room temperature for 5 hours under the action of a magnetic stirrer to obtain a brick The red precipitate product was then dripped with 6.0mL H at a rate of 1.5mL / min 2 o 2 After 20 minutes of reaction, 4.1960 g of lithium hydroxide was added thereto, stirred for 12 hours, and then the cloudy solution was transferred to a polytetrafluoroethylene-lined reactor, and reacted at a constant temperature of 130° C. for 12 hours. The resulting precipitate was filtered, washed, and dried to obtain a reddish-brown intermediate product, which was ground to uniform and fine particles, then placed in a muffle furnace at a rate of 10°C / min to 500°C, kept at a constant temperature for 5 hours, and then cooled naturally to room temperature, the spinel li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com