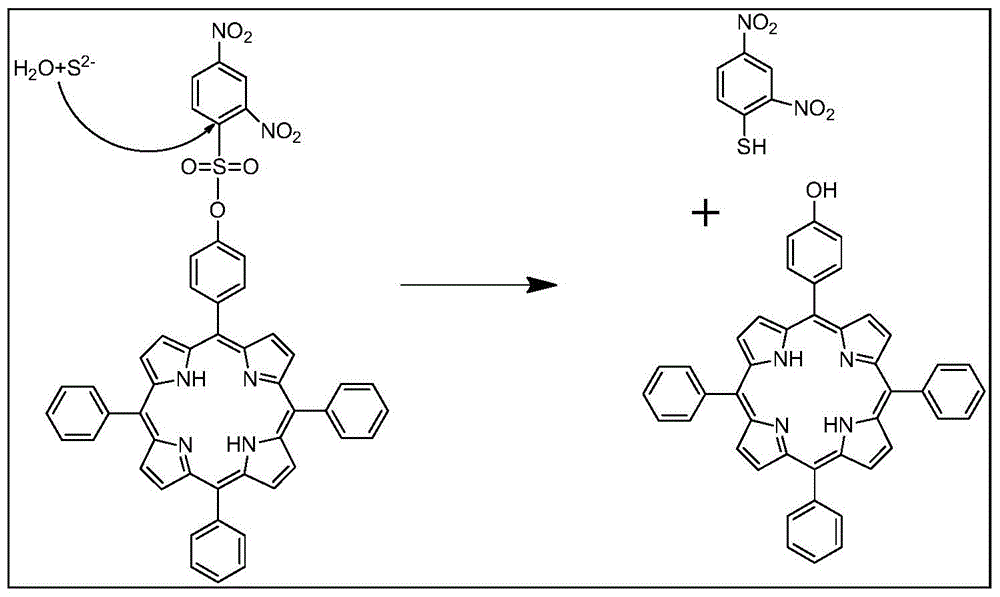
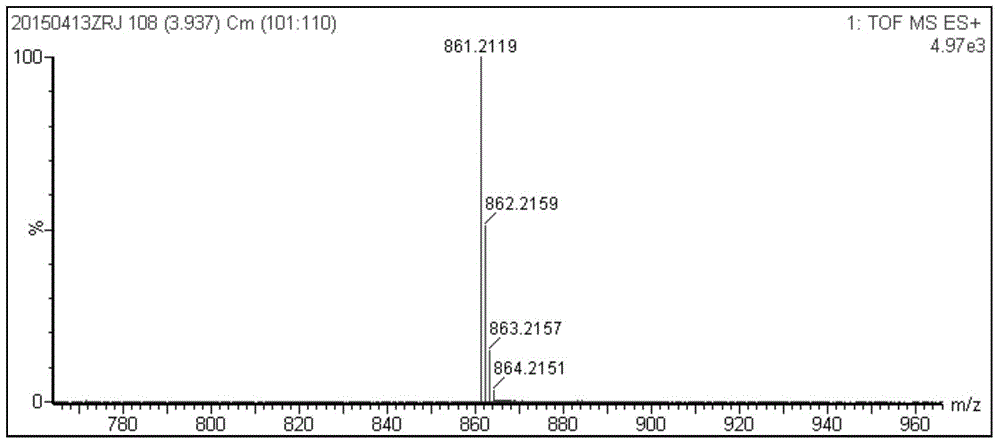
Synthetic method and application of porphyrin type near-infrared sulfur ion fluorescence probe
A technique for fluorescent probes and synthesis methods, applied in the field of synthesis of porphyrin-type near-infrared fluorescent probes, to achieve the effects of reducing background fluorescence interference, simple synthesis routes, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of synthetic method of porphyrin type near-infrared sulfur ion fluorescent probe (monohydroxyphenylporphyrin-2,4-dinitrobenzenesulfonate), specifically comprises the following steps:
[0031] (1) Weigh A (monohydroxyphenylporphyrin (30mg, 0.048mmol) and dissolve it in 10ml of refined dichloromethane, then add DIEA (0.019ml, 0.116mmol), then weigh B (2,4-dinitro Benzenesulfonyl chloride (17.7mg, 0.066mmol) was dissolved in 10ml of refined dichloromethane, filled with N 2 After protecting for 20 to 30 minutes, use a constant pressure dropping funnel to drop the solution of B into A, and stir at room temperature for 24 to 36 hours to obtain dichloromethane of monohydroxyphenylporphyrin-2,4-dinitrobenzenesulfonate solution;
[0032] (2) the dichloromethane solution of monohydroxyphenylporphyrin-2,4-dinitrobenzenesulfonyl ester is washed 3 to 4 times with 20ml saturated sodium chloride solution, and the organic layer is dried with anhydrous sodium sulfate ( Stir for...
Embodiment 2
[0034] Sulfur ion detection experiment.
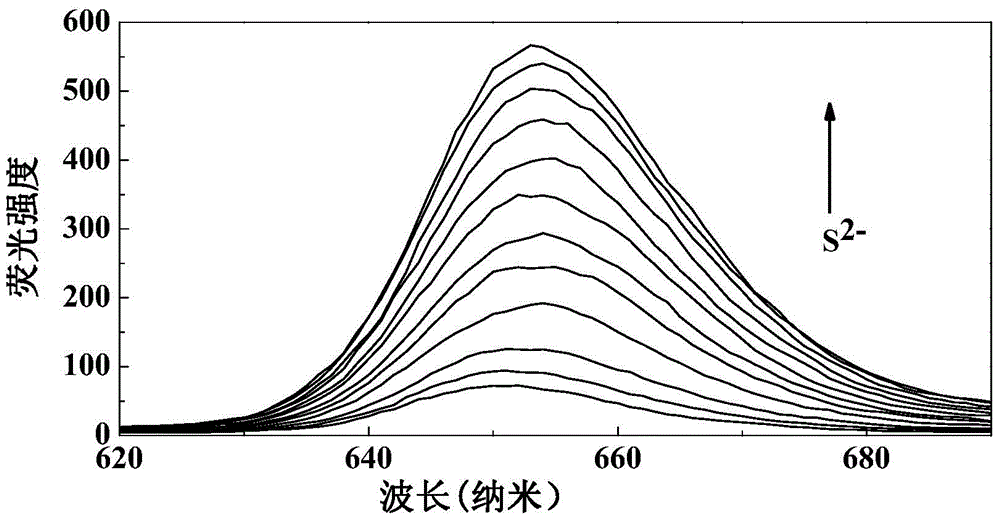
[0035] (1) Add 5.0ml of THF, 1.16mmol L to a 10ml volumetric flask -1 Probe solution 86 μl, pH 7.4 0.2mol.L -1 0.5ml of phosphate buffer solution, diluted to 10ml with twice distilled water, to obtain a concentration of 10μmol L -1 probe solution. Take 3.0ml probe solution and add it into 4ml cuvette, add S 2- , so that the concentrations are 0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10 μmol L -1 . Stir the reaction at room temperature for 8 minutes to measure its fluorescence emission spectrum (λ ex =550nm,λ em = 570nm). to obtain probes with different concentrations of S 2- The fluorescence emission spectrum of the reaction (see image 3 ). Depend on image 3 It can be seen that the fluorescence intensity of the probe solution itself is very small, when adding S 2- After 8 minutes of reaction, the fluorescence intensity of the reaction system was significantly enhanced, and with the S 2- As the concentration inc...
Embodiment 3
[0038] Sulfide ion selectivity experiment.
[0039] (1) Add 5.0ml THF, 1.16mmol.L, to a 10ml volumetric flask -1 Probe solution 86 μl, pH 7.4 0.2mol.L -1 0.5ml of phosphate buffer solution, diluted to 10ml with twice distilled water, to obtain a concentration of 10μmol L -1 Probe solution. Take 3.0ml probe solution and add it into 4ml cuvette, add anion: S 2- , I - , Cl - , Br - ,C 2 o 4 2- , SO 3 2- , HSO 3 - , F - ,NO 2 - , Ac - , SO4 2- ,NO 3 - , S 2 o 3 2- , CO 3 - , SCN - , HCO 3 - , so that the concentration is 5μmol.L -1 , measured its fluorescence intensity (λ ex =550nm;λ em =570nm), obtain the fluorescence emission spectrum figure after probe reacts with different anions (see Figure 5 ). Depend on Figure 5 It can be seen that adding S 2- After adding the same concentration of other anions, the fluorescence intensity was significantly enhanced, but the fluorescence intensity was almost unchanged after adding the same concentration of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com